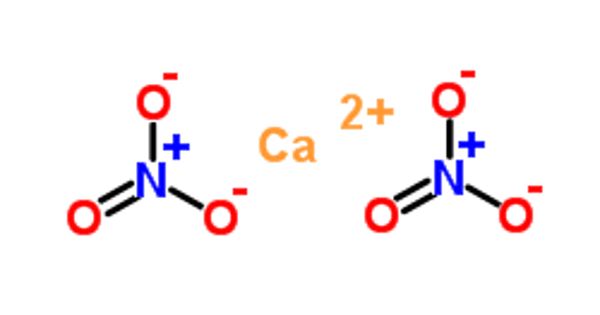
Calcium nitrate is a nitrate salt of Calcium which contains calcium and nitrogen and oxygen. It is also called Norgessalpeter (Norwegian saltpeter), is an inorganic compound with the formula Ca(NO3)2. It appears as white to light gray granular solid. This colourless salt absorbs moisture from the air and is commonly found as a tetrahydrate. It can be applied to soils and is a source of calcium and nitrate in greenhouse production and in hydroponics. It is mainly used as a component in fertilizers but has other applications.
Calcium nitrate is inorganic nitrate salt of calcium. It has a role as a fertilizer. It is an inorganic nitrate salt and a calcium salt.
Nitrocalcite is the name for a mineral which is a hydrated calcium nitrate that forms as an efflorescence where manure contacts concrete or limestone in a dry environment as in stables or caverns. It is a white or whitish-grey coloured granular solid which absorbs moisture from the air and is usually found as a tetrahydrate compound. A variety of related salts are known including calcium ammonium nitrate decahydrate and calcium potassium nitrate decahydrate.

Properties
It is a colorless crystalline deliquescent salt occurring often in natural waters and soil and as the mineral nitrocalcite, made by reaction of nitric acid or nitrogen oxides with lime or calcium carbonate, and used as a fertilizer.
- Molecular Weight: 164.09
- Appearance: White Powder
- Melting Point: 42 °C
- Boiling Point: 132 °C (dec.)
- Density: 2.5 g/cm3
- Solubility in H2O: 1293 g/L (20 °C)
- Exact Mass: 163.938
- Monoisotopic Mass: 163.938
Production and reactivity
Norgessalpeter was synthesized at Notodden, Norway in 1905 by the Birkeland–Eyde process. Most of the world’s calcium nitrate is now made in Porsgrunn.
Calcium nitrate is produced by applying nitric acid to limestone and then adding ammonia. It is produced by treating limestone with nitric acid, followed by neutralization with ammonia:
CaCO3 + 2 HNO3 → Ca(NO3)2 + CO2 + H2O
It is also an intermediate product of the Odda Process:
Ca5(PO4)3OH + 10 HNO3 → 3 H3PO4 + 5 Ca(NO3)2 + H2O
Calcium nitrate is also formed when an ammonium nitrate solution is mixed with a calcium hydroxide solution. It can also be prepared from an aqueous solution of ammonium nitrate, and calcium hydroxide:
2 NH4NO3 + Ca(OH)2 → Ca(NO3)2 + 2 NH4OH
Like related alkaline earth metal nitrates, calcium nitrate decomposes upon heating (starting at 500 °C) to release nitrogen dioxide:
2 Ca(NO3)2 → 2 CaO + 4 NO2 + O2 ΔH = 369 kJ/mol
It is known as a double salt, since it is comprised of two nutrients common in fertilizers which are high in sodium. The processed result also looks crystallized like salt. It is not organic and is an artificial fertilizer amendment.
Uses
Calcium nitrate fertilizer can be used as a foliar spray. Its fertilizer is often a go-to choice of fertilizers to produce larger vegetables, strong plants, and faster growth. It is used in fertilizers, explosives and pyrotechnics.
Information Source: