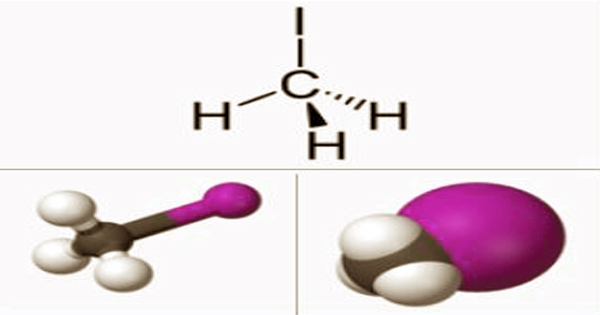
Iodomethane, also known as methyl iodide and abbreviated “MeI,” is a kind of iodomethane that is formed when one of the hydrogens in methane is replaced by iodine. It’s a chemical compound, and it’s a thick, colorless, volatile liquid with the formula CH3I. It’s a volatile liquid that’s related to methane but without hydrogen. Iodomethane dissolves in a variety of organic solvents. It is linked to methane in chemical structure because one hydrogen atom is replaced by an iodine atom.
Iodomethane is colorless, however, samples take on a reddish hue when exposed to light. Rice crops naturally emit modest amounts of it. Algae and kelp in the world’s temperate oceans create huge amounts of it, estimated to be more than 214,000 tons per year, and terrestrial fungus and bacteria create smaller amounts on land. Iodomethane (methyl iodide) is commonly employed in chemical synthesis to deliver a methyl group through the methylation process.
It has a role as a fumigant insecticide. It belongs to the methyl halides and the iodomethanes families. It is utilized as a source of methyl groups in chemical synthesis. In 2007, the United States Environmental Protection Agency approved iodomethane for use as a pre-plant biocide to reduce insects, plant parasitic nematodes, soil borne diseases, and weed seeds in vegetables such as. Skin, eyes, and mucous membranes may be irritated by contact. Ingestion, inhalation, and skin absorption are all extremely dangerous.
When iodine is added to a mixture of methanol and red phosphorus, an exothermic reaction occurs, yielding iodomethane. It’s approved for use on field-grown strawberries, peppers, nut crops, tomatoes, grape vines, ornamentals, and turf, as well as nursery-grown strawberries, stone fruits, tree nuts, and conifer trees. The iodinating reagent is phosphorus triiodide that is formed in situ:
3 CH3OH + PI3 → 3 CH3I + H2PO3H
It is non-corrosive to metals, stable at room temperature in sealed containers, and incompatible with strong oxidizing and reducing agents. The reaction of dimethyl sulfate with potassium iodide in the presence of calcium carbonate produces iodomethane:
(CH3O)2SO2 + KI → CH3I + CH3OSO2OK
Methanol and aqueous hydrogen iodide can also be combined to make it:
CH3OH + HI → CH3I + H2O
Due to disintegration and the escape of free iodine, the color changes yellow, red, or brown when exposed to light and moisture. Toxic iodine fumes are released when heated to breakdown in air at 270 degrees. Iodomethane can also be made by combining iodoform with potassium hydroxide and dimethyl sulfate in a 95% ethanol solution. It can be synthesized by the following several methods:
- The reaction between methanol with phosphorus triiodide.
- In the presence of calcium carbonate, dimethyl sulfate reacts with potassium iodide.
- The reaction of methanol with aqueous hydrogen iodide.
- In the presence of ethanol, the iodoform reacts with potassium hydroxide and dimethyl sulfate.
Iodomethane, like many other organoiodide chemicals, is normally maintained in dark bottles to prevent light-induced iodine degradation, which results in a purplish tint to deteriorated samples. Copper or silver wire can be used to stabilize commercial samples. Its use as a soil fumigant is set to end, and iodomethane has been recommended as a replacement. Microbial methylation of iodide produces the majority of iodomethane. Oceans are the primary supply, although rice paddies play an important role as well.
In the production of several medications and pesticides, iodomethane is employed as an intermediary. It’s also employed in the areas of microscopy and methylation processes. Nucleophiles can attack it sterically, and iodide is a suitable leaving group. It’s utilized to alkylate nucleophiles of carbon, oxygen, sulfur, nitrogen, and phosphorus. Methyl iodide has been proposed as a fire extinguisher and an insecticidal fumigant.
Iodomethane is a valuable methylating agent that can be used to alkylate nucleophiles such as carbon, oxygen, sulfur, nitrogen, and phosphorus. Iodides are often more expensive than chlorides and bromides, however iodomethane is very inexpensive; on a commercial scale, the more poisonous dimethyl sulfate is favored since it is less expensive and has a higher boiling point. It forms the Grignard reagent, methyl-magnesium iodide, which is utilized in chemical synthesis, when it combines with magnesium.
It’s also used as a phase-transfer catalyst and as an intermediary in the production of medications. Iodomethane’s iodide leaving group may induce undesired side effects. Finally, because of its strong reactivity, iodomethane poses a greater risk to laboratory personnel than chlorides and bromides. It’s employed as an intermediary in the production of various medications and pesticides, as well as in methylation and microscopy.
Iodomethane and other organic iodine compounds do occur in the aftermath of a major nuclear disaster, as evidenced by the detection of iodine-131 in the form of organic iodine compounds in Europe and Japan, respectively, following the Fukushima and Chernobyl disasters. It can also be used as a pesticide in the vegetable industry to suppress insects, plant parasitic nematodes, soil transmitted diseases, and weed seeds. It can be used to grow strawberries, peppers, nut crops, tomatoes, grape vines, ornamentals, and turf in the field, as well as strawberries, stone fruits, tree nuts, and conifer trees in the nursery.
Ingestion, inhalation, and skin contact all have a moderate acute toxicity to methyl iodide. Because this chemical is easily absorbed through the skin, it has the potential to induce systemic toxicity. Metabolic disruption, renal failure, venous and arterial thrombosis, and encephalopathy with seizures and coma, with a distinctive pattern of brain injury, are all symptoms of high dose acute poisoning, which can occur in industrial accidents. At high temperatures, high vapor pressure can cause containers to burst.
Information Sources: