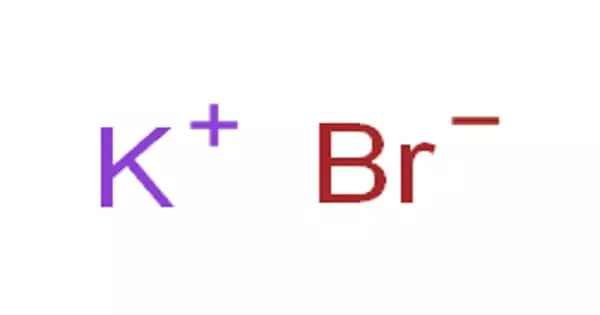Potassium bromide (KBr) is a salt that was widely used as an anticonvulsant and sedative in the late nineteenth and early twentieth centuries, with over-the-counter use in the United States dating back to 1975. It is also known as a potassium bromide salt, kalii bromidum, and tripotassium tribromide. The bromide ion is responsible for its action (sodium bromide is equally effective). It appears in the form of odorless colorless crystals, white crystalline powder, or white granular solid with a pungent bitter saline taste.
Under normal conditions, potassium bromide is a pure, crystalline powder. It is freely soluble in water, whereas acetonitrile is not. In a dilute aqueous solution, it tastes sweet, sour at low concentrations, and salty at very low concentrations.
Potassium bromide is a veterinary drug that is used to treat epilepsy in dogs. The potassium bromide salt is odorless and comes in the form of white crystalline powder, colorless crystals, or white granular solid with a pungent bitter saline taste.
Properties
Potassium bromide is a white crystalline powder under normal conditions. It is freely soluble in water but not in acetonitrile. Potassium bromide tastes sweet in a dilute aqueous solution, bitter at higher concentrations, and salty at even higher concentrations. These effects are primarily due to the properties of the potassium ion—at any concentration, sodium bromide tastes salty. Potassium bromide, in high concentrations, irritates the gastric mucous membrane, causing nausea and vomiting (a typical effect of all soluble potassium salts).
- Molecular weight: 119.002 g/mol
- Density: 2.74 g/cm3
- Melting point: 734 °C
- Boiling point: 1,435 °C
- Appearance: White Powder
- Solubility in H2O: 53.48 g/100 g H2O (293K)

Chemical properties
Potassium bromide, a typical ionic salt, is fully dissociated and near pH 7 in an aqueous solution. It serves as a source of bromide ions. This reaction is important for the manufacture of silver bromide for the photographic film:
KBr(aq) + AgNO3(aq) → AgBr(s) + KNO3(aq)
Aqueous bromide Br− also forms complexes when reacted with some metal halides such as copper(II) bromide:
2 KBr(aq) + CuBr2(aq) → K2[CuBr4](aq)
Preparation
The reaction of potassium carbonate with an iron(III, II) bromide, Fe3Br8, produced by treating scrap iron in water with excess bromine is a traditional method for producing KBr:
4 K2CO3 + Fe3Br8 → 8 KBr + Fe3O4 + 4 CO2
The most common industrial process for producing KBr is the reaction of potassium carbonate or K2CO3 and iron (III, II) bromide or Fe3Br8. Underwater, scrap irons with extra bromine are used in this reaction.
Reactions
It is a typical ionic salt that is fully dissociated at a near pH value of 7 in the aqueous solution. This reaction plays an important role in the manufacture of silver bromide for photographic films. The reaction is as follows:
KBr(aq) + AgNO3(aq) → AgBr(s) + KNO3(aq)
Bromide in its aqueous form produces complexes on reacting with metal halides like copper (II) bromide:
2 KBr(aq) + CuBr2(aq) → K2[CuBr4](aq)
Uses
- Potassium Bromide is used to manufacture photographic papers and plates.
- Used as a laboratory agent.
- Used as heat stabilizer for nylon.
- Used as a Sedative.
- Used as an anticonvulsant.
- Used in the water treatment of aquariums
- Used to manufacture chemicals.
- Used as plasticizers.
Health Hazards
Vomiting, ataxia, coma, irritability, and mental confusion are among the symptoms. Mania, skin rashes, drowsiness, and hallucinations are all possible side effects. It also results in neurological symptoms, increased spinal fluid pressures, death, vertigo, and sensory disturbances.