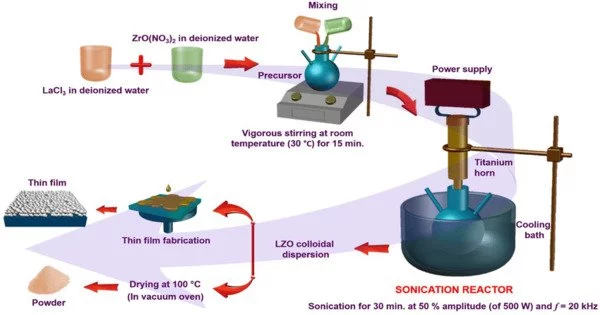
Sonochemistry is a branch of chemistry concerned with the investigation of chemical reactions and processes influenced by ultrasound waves. Sonochemistry is the study of the effect of ultrasound in forming acoustic cavitation in liquids, which results in the initiation or enhancement of chemical activity in the solution. As a result, the chemical effects of ultrasound are not the result of a direct interaction between the ultrasonic sound wave and the molecules in the solution.
Ultrasound refers to sound waves with frequencies greater than the upper limit of human hearing, typically greater than 20,000 hertz (Hz). These high-frequency sound waves are used in sonochemistry to cause physical and chemical changes in substances.
Sonochemistry finds applications in various fields of chemistry, including organic synthesis, inorganic chemistry, and materials science. Some common applications of sonochemistry include:
- Organic Synthesis: Ultrasound can speed up organic reactions and increase yields. It is used in processes such as nanoparticle synthesis, biodiesel production, and pollutant degradation.
- Catalysis: By providing mechanical agitation and promoting better reactant mixing, sonochemistry can improve the efficiency of catalytic reactions.
- Materials Science: Ultrasound can have an impact on the morphology and properties of materials. It is used to make nanomaterials, emulsions, and other advanced materials.
- Extraction: Ultrasound-assisted extraction is used to extract compounds more efficiently from plants, foods, and other natural sources than traditional methods.
- Analytical Chemistry: Ultrasound is employed in analytical techniques, such as sample preparation and cleaning of analytical instruments.
Application
The use of ultrasound in chemistry can result in a variety of effects such as cavitation, microstreaming, and increased mass transfer. Cavitation is the formation, growth, and collapse of bubbles in a liquid, which causes energy to be released and localized high temperatures and pressures to be created. This phenomenon can result in the formation of highly reactive species like free radicals, which can speed up chemical reactions.
The effects of ultrasound in sonochemistry are complex and depend on a number of factors, such as the frequency and intensity of the ultrasound, the nature of the reactants, and the solvent used. Researchers continue to investigate and optimize sonochemical processes for a wide range of chemistry applications.