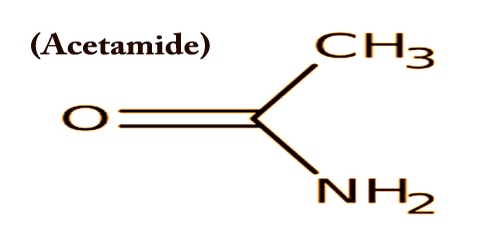
Acetamide (systematic name: ethanamide) is also called Acetic acid amide, or Ethanamide or Acetimidic acid; is an organic compound with the formula CH3CONH2. It is a member of the class of acetamides arising from the formal condensation of ammonia to acetic acid. It has some use as a plasticizer and as a solvent for the industry. As a plasticizer it is commonly used. The related compound N,N-dimethylacetamide (DMA) is more widely used, but it is not prepared from acetamide.
Acetamide can be considered an intermediate between acetone, which has two methyl (CH3) groups either side of the carbonyl (CO), and urea which has two amide (NH2) groups in those locations. Acetamide with a mousy odor appears as colorless crystals. It is readily soluble and partially soluble in the ether in water, chloroform, hot benzene, and glycerol.
In chemical laboratories, Acetamide can be produced by dehydration of ammonium acetate. The reaction is as follows:
(NH4)(CH3CO2) → CH3C(O)NH2 + H2O
Alternatively, acetamide may be obtained in excellent yield via ammonolysis of acetylacetone under conditions commonly utilized in reductive amination. Alternately, it will be produced from anhydrous acetic acid (CH3COOH), dried acid gas, and acetonitrile in an ice bath together with a reagent acyl halide.
In a similar fashion to some laboratory methods, acetamide is produced by dehydrating ammonium acetate or via the hydration of acetonitrile, a byproduct of the production of acrylonitrile:
CH3CN + H2O → CH3C(O)NH2
Acetamide is combustible, and when heated produces poisonous gas or fumes. Exposure to amide ethanoic acid can irritate the mucous membranes, skin, and eyes. It can cause damage to the cornea too. Molten acetamide is nice solvent with a broad range of applicability; notably, its dielectric constant is above most organic solvents, allowing it to dissolve inorganic compounds with solubilities closely analogous thereto of water.
Acetamide is found in red beetroot; it’s produced by dehydrating ammonium acetate. This can be the only amide of carboxylic acid derivatives. Acetamide is employed within the manufacture of polymeric products, like polyvinyl acetamide, a polymeric commodity used as an absorbent, as a co-monomer. It’s a precursor to thioacetamide.
Acetamide has low acute toxicity in laboratory animals; the only toxic effect associated with one single exposure to a very high oral dose was reduced body weight. Acetamide was found close to the Milky Way galaxy center; this finding is potentially significant because acetamide has an amide bond, similar to the essential bond between amino acids in proteins. Moreover, acetamide, as a mineral of the same name, is rarely found on burning coal dumps.
Information Sources: