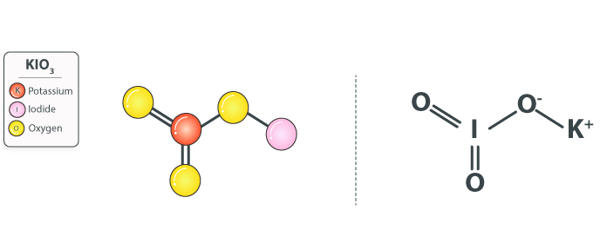
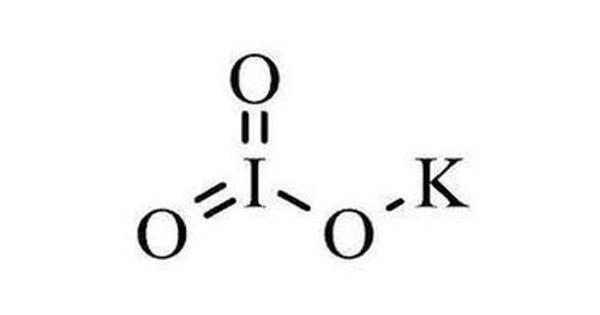Potassium iodate (KIO3) is an oxidizing agent and as such, it can cause fires if in contact with combustible materials or reducing agents. It is an ionic chemical compound consisting of K+ ions and IO3– ions in a 1:1 ratio. It can be prepared by reacting potassium base with iodic acid.
Potassium iodate is the major chemical compound used for the iodization of edible common salts. It provides a convenient way of performing iodometric work. Potassium iodate is also sometimes used to precipitate thorium especially to remove it from the rare earth.
Properties
Potassium iodate is an oxidizing agent and as such it can cause fires if in contact with combustible materials or reducing agents.
- Density: 3.89 g/cm³
- Molecular Weight/ Molar Mass: 214.001 g/mol
- Specific gravity: 4.93 g/cm3
- Melting Point: 560 °C
- Appearance: White crystalline powder
- Solubility: Soluble in KI solution insoluble in alcohol
Preparation
Potassium iodate is produced by reacting potassium hydroxide to iodine acid or by reacting potassium hydroxide to iodine. It can be prepared by reacting a potassium-containing base such as potassium hydroxide with iodic acid, for example:
HIO3 + KOH → KIO3 + H2O
It can also be prepared by adding iodine to a hot, concentrated solution of potassium hydroxide.
3 I2 + 6 KOH → KIO3 + 5 KI + 3 H2O
Potassium iodate combines with potassium iodide in the presence of strong acid like sulfuric acid forms potassium sulfate, iodine and water.
KIO3 + 5KI + 3H2SO4 → 3K2SO4 + 3H2O + 3I2
Or by fusing potassium iodide with potassium chlorate, bromate or perchlorate, the melt is extracted with water and potassium iodate is isolated from the solution by crystallization:
KI + KClO3 → KIO3 + KCl
Potassium iodate is an oxidizing agent and in contact with combustible materials or reducing agents, it can cause fires as well. Conditions/substances to avoid include: heat, shock, friction, combustible materials, reducing materials, aluminium, organic compounds, carbon, hydrogen peroxide and sulfides.
Applications
- Potassium iodate is sometimes used for iodination of table salt to prevent iodine deficiency.
- It is used in the iodination of table salt because iodide can be oxidised by molecular oxygen to iodine under wet conditions.
- Like potassium bromate, potassium iodate is occasionally used as a maturing agent in baking
- It is used in the analysis of testing arsenic and zinc.
- It is used in the iodometry in medicine manufacturing.
- It is used as a reagent and as feed additives.
Toxicity
Potassium iodate can produce retinal toxicity that damages RPE and photoreceptor cells. The recovery of retinal function depends on the amount of chemical absorption, the regeneration of RPE, and the recovery function of photoreceptor cells.
Information Source: