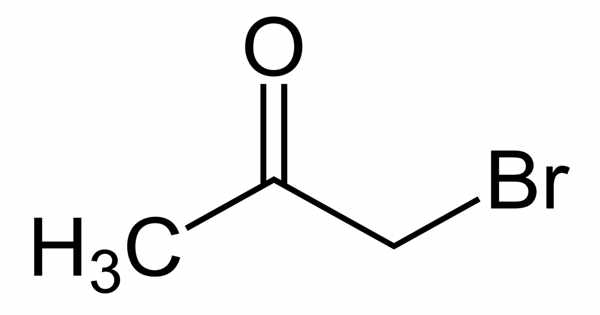
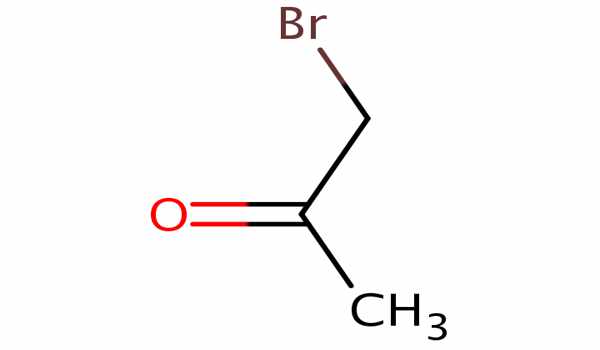
The organic compound bromoacetone has the formula CH3COCH2Br. It appears as a clear colorless liquid that turns violet when left standing, even in the absence of air, and decomposes to a black resinous mass after a long period of time. This colorless liquid functions as a lachrymatory agent as well as a precursor to other organic compounds. It is a lachrymatory agent that was used as a chemical weapon during World War I and later as a riot-control agent.
Properties
Bromoacetone is an alpha-bromoketone, which is acetone in which one of the hydrogen atoms has been replaced by a bromine atom. It has a higher density than water and is less soluble in water. As a result, it sinks in water. It has been demonstrated that it is a byproduct of drinking water treatment with chlorine dioxide and chloramines in bromide-rich water.
- Appearance: Colorless Liquid
- Boiling Point: 137°C
- Density: 1.63 g/cm3
- Melting Point: -36.5 °C
- Molar Mass: 136.99 g/mol
- Solubility: Soluble
Occurrence In nature
Bromoacetone occurs naturally (less than 1% ) in the essential oil of a seaweed (Asparagopsis taxiformis) from the Hawaiian Islands. It can be found in the essential oil of a seaweed called Asparagopsis taxiformis, which grows in the ocean around the Hawaiian Islands.
Synthesis
Bromoacetone is commercially available, and it is sometimes stabilized with magnesium oxide. It was first described in the nineteenth century by N. Sokolowsky.
Bromoacetone is made by combining bromine, acetone, and catalytic acid. Acetone, like all ketones, enolizes in the presence of acids or bases. The alpha carbon is then electrophilically substituted with bromine. The main drawback of this method is over-bromination, which results in di- and tribrominated products. If a base is present, the haloform reaction produces bromoform instead.
Applications
It was used as a chemical weapon during World War I, and was known as BA by the British and B-Stoff (Weisskreuz) by the Germans. It is no longer used as a riot-control agent due to its toxicity. Bromoacetone is a versatile organic synthesis reagent. By reacting with aqueous sodium hydroxide, it can be converted to hydroxyacetone.
Health Risks
Low concentrations are very irritating to the eyes; high concentrations or prolonged exposure at lower concentrations may have negative health effects. It is extremely toxic when inhaled. The liquid causes painful burns when it comes into contact with it. Used as a chemical warfare agent.