The oxygen reduction reaction (ORR) is an electrochemical process that involves the reduction of oxygen molecules to water in various energy conversion devices, such as fuel cells and metal-air batteries. The ORR is a crucial reaction in these devices because it determines their efficiency and performance.
A research group led by Prof. Wang Liping at the Ningbo Institute of Materials Technology and Engineering (NIMTE) of the Chinese Academy of Sciences has reported the enhanced oxygen reduction reaction (ORR) activity and biperiodic chemical trends of lanthanide-doped molybdenum disulfide (Ln-MoS2). The study was published in Nature Communications.
Lanthanide-doped molybdenum disulfide (MoS2) refers to the incorporation of lanthanide elements into the crystal lattice of MoS2, resulting in modified properties and potential applications.
MoS2 offers a wide range of potential applications in the disciplines of optoelectronics, solid lubrication, and catalysis, among others. Various lanthanides (Ln), such as Sm, Eu, Dy, Ho, Er, and Yb, etc., can be doped into MoS2 to modify its physicochemical properties. However, it’s important to note that specific biperiodic trends in lanthanide-doped MoS2 can depend on various factors such as the lanthanide element used, the doping concentration, and the synthesis method employed.
The performance and longevity of Ln-MoS2-based functional materials, coatings, and devices, including the fuel cell efficiency and device galvanic corrosion, are significantly influenced by surface oxygen reduction, which converts O2 to H2O.
Exploring the ORR activity on the Ln-MoS2 surface and its orbital chemistry mechanism can offer suggestions for the design of practical applications, accurate performance regulation, and efficient Ln-MoS2 system protection.
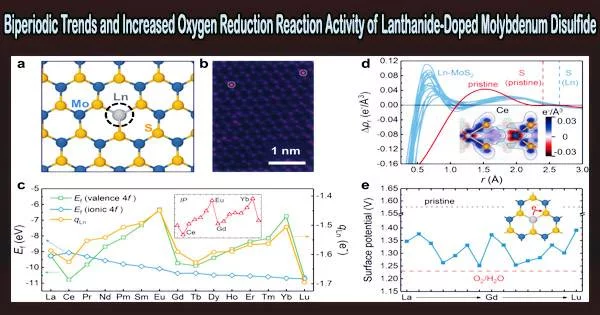
Through density-functional theory calculations, the researchers investigated the ORR process on all the 15 Ln-MoS2 (Ln = La ~ Lu) surfaces.
The doping of Ln significantly enhanced the ORR activity on Ln-MoS2 surfaces. In addition, a fascinating modulating biperiodic chemical trend of the ORR activity was observed.
Furthermore, using thermodynamic statistics, the water effect on the loose f/liquid interface was accurately modelled. To accurately direct the associated experiments and quantitatively disclose the ORR activity, current-potential polarization curve simulations were also carried out.
A defect-state pairing mechanism, which selectively stabilizes the hydroxyl and hydroperoxyl adsorbates on Ln-MoS2, is responsible for the increased ORR activity, according to a thorough electronic structure investigation. This process also dramatically lowers the ORR energy barrier.
Additionally, a general theory of the orbital chemistry of Ln-MoS2 systems was put out, which contributes to the explanation of the intrinsic biperiodic patterns seen in a variety of electronic, thermodynamic, and kinetic parameters.
This research provides information on the development of excellent Ln-MoS2-based materials and related systems with promising commercial potential for use as anti-corrosion coatings, optoelectronic nanodevices, and electrocatalysts.