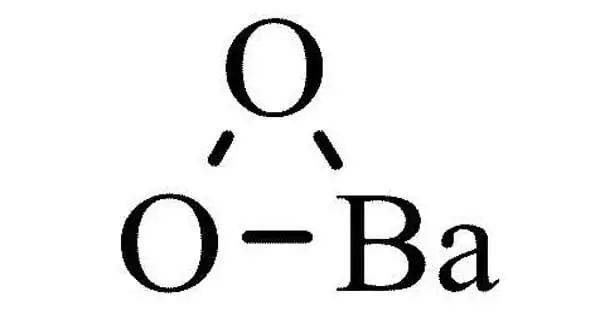The inorganic chemical with the formula BaO2 is barium peroxide. It has the appearance of a grayish-white granular solid. This white solid (gray when impure) is the first peroxide chemical identified and is one of the most prevalent inorganic peroxides. As a result, before use, barium peroxide is always diluted to make a slurry. Barium peroxide is a powerful oxidizing agent used in bleaching.
It’s not soluble in water. It is noncombustible, however it speeds up the combustion of combustible material. Because it is an oxidant and emits a bright green flame when ignited (as do other barium compounds), it is used in pyrotechnics; historically, it was also employed as a precursor for hydrogen peroxide. When combined with water, it releases its bleaching properties.
Properties
BaO2 is an iron gray or white powder. It is slowly decomposed in air, forming hydroxide and oxygen. It does not dissolve in water, but can slowly hydrolyze, forming hydrogen peroxide in solution. It is a strong oxidizing agent and will explode if direct contact with organic matter occurs.
- Molecular Weight: 169.34
- Appearance: White, Cream, or Grayish Powder
- Melting Point: 450 °C
- Boiling Point: 800 °C (decomposes)
- Density: 4.96 g/cm3
- Solubility in H2O: N/A
Structure
Barium peroxide is a peroxide, containing O22- subunits. The solid is isomorphous to calcium carbide, CaC2. It a compound BaO2 obtained as a grayish-white toxic powder by heating barium monoxide in air or oxygen and is used chiefly in making hydrogen peroxide and in pyrotechnics.

Preparation and use
Barium peroxide arises by the reversible reaction of O2 with barium oxide. The peroxide forms around 500 °C and oxygen is released above 820 °C.
2 BaO + O2 ⇌ 2 BaO2
This reaction is the basis for the now-obsolete Brin process for separating oxygen from the atmosphere. Other oxides, e.g. Na2O and SrO, behave similarly.
In another obsolete application, barium peroxide was once used to produce hydrogen peroxide via its reaction with sulfuric acid:
BaO2 + H2SO4 → H2O2 + BaSO4
The insoluble barium sulfate is filtered from the mixture.
To obtain metal peroxides, the reaction of the corresponding oxide is usually carried out with hydrogen peroxide in the corresponding solvent.
In the case of barium peroxide, it is no exception, and a reversible reaction of the barium oxide with molecular oxygen takes place, so that the peroxide forms around 500 °C, and oxygen is released from the 820 °C.
Application
Barium peroxide is primarily used in bleaching, thermal welding of aluminum, as an oxidizing agent, and textile dyeing.
It is utilized as a source of hydrogen peroxide, an oxygen oxidant, and a bleaching agent. Its primary applications have been in the production of hydrogen peroxide and oxygen, as well as in organic synthesis, fabric printing, and dyeing. It is commercially accessible, primarily as the oxctahydrate.