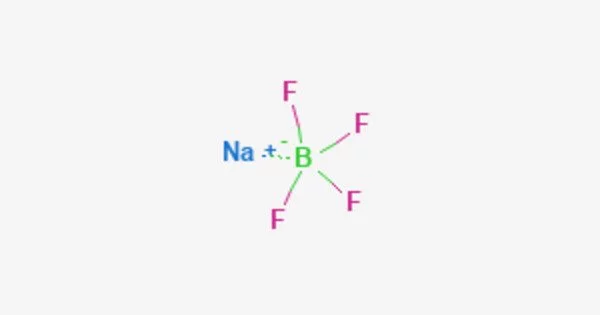
Sodium tetrafluoroborate (NaBF4) is an inorganic compound with the chemical formula NaBF4. It is a salt that forms colorless or white water-soluble rhombic crystals and is soluble in water (108 g/100 mL) but less soluble in organic solvents. It is a colorless or white crystalline powder that is soluble in water and ethanol. It is a useful and versatile compound with a variety of applications in chemistry and industry.
Sodium tetrafluoroborate is used in some fluxes used for brazing and to produce boron trifluoride. It is generally considered safe to handle, but it can release hydrogen fluoride gas under certain conditions, which is highly toxic and corrosive.
Properties
NaBF4 is stable at room temperature but can decompose at high temperatures or under acidic conditions, releasing hydrogen fluoride gas. It is an ionic compound, and in aqueous solutions, it dissociates into sodium cations (Na+) and tetrafluoroborate anions (BF4-). It is a strong Lewis acid and can be used as a catalyst in various reactions.
- Chemical formula: NaBF4
- Molar mass: 109.794 g/mol
- Density: 2.47 g/cm3
- Melting point: 384 °C (723 °F; 657 K)
Preparation
Sodium tetrafluoroborate can be prepared by neutralizing tetrafluoroboric acid with sodium carbonate or sodium hydroxide.
NaOH + HBF4 → NaBF4 + H2O
Na2CO3 + 2 HBF4 → 2 NaBF4 + H2O + CO2
Alternatively the chemical can be synthesized from boric acid, hydrofluoric acid, and sodium carbonate:
2H3BO3 + 8HF + Na2CO3 → 2NaBF4 + 7H2O + CO2
Reactions
On heating to its melting point, sodium tetrafluoroborate decomposes to sodium fluoride and boron trifluoride:
NaBF4 → NaF + BF3
It is a source of tetrafluoroborate anion, which is used in organic chemistry for the preparation of salts. Sodium tetrafluoroborate can be used for synthesis of ionic liquids, where tetrafluoroborate is the anion.
Application
NaBF4 is commonly used as a source of fluoride ions in organic and inorganic synthesis, as well as in electroplating, metal finishing, and as an electrolyte in batteries. It is also used as a reagent in analytical chemistry for the determination of trace amounts of mercury in water and other environmental samples.
In addition, NaBF4 is used as a fluorinating agent in organic synthesis, where it can replace other fluorine-containing reagents that are more toxic or difficult to handle. It is also used as a component in some dental products, such as fluoride varnishes and gels.
Toxicity
NaBF4 is generally considered to be safe and non-toxic when handled properly. However, it is important to follow proper handling and disposal procedures to avoid any potential hazards associated with the compound.