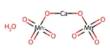
Ammonium heptamolybdate is an inorganic substance with the chemical formula (NH4)6Mo7O24 that is commonly found as a tetrahydrate. A dihydrate is also referred to. It is a colorless solid that is commonly referred to as ammonium paramolybdate or simply ammonium molybdate, while “ammonium molybdate” can also refer to ammonium orthomolybdate, (NH4)2MoO4, and a number of other compounds. It is a rather common molybdenum compound.
It is a crystalline substance with no odor. It is used as an analytical reagent to determine the concentrations of phosphates, silicates, arsenates, and lead in aqueous solutions. It is also employed in the manufacture of molybdenum metal and ceramics, the creation of dehydrogenation and desulphurization catalysts, metal fixing, electroplating, agricultural fertilizers, and as a negative stain in biological electron microscopy.
Properties
It is a colorless or light yellow-green columnar crystals. Soluble in 2.3 water, insoluble in ethanol, acid decomposition. When heated to 90 °c, one molecule of crystal water is lost and decomposed when heated to 190 °c. The pH value of 5% aqueous solution is 5.O ~ 5.5. The relative density was 2. 498.
- Molecular Weight: 1163.9
- Appearance: White to off-white powder or crystals
- Melting Point: 190 °C
- Boiling Point: N/A
- Density: 2.498 g/cm3
- Solubility in H2O: N/A

Synthesis
Ammonium heptamolybdate is easily made by dissolving molybdenum trioxide in sufficient aqueous ammonia and evaporating the solution at ambient temperature. The ammonia excess exits as the solution evaporates. The six-sided translucent prisms of ammonium heptamolybdate tetrahydrate are formed as a result of this approach.
Molybdic acid and an ammonium salt are formed when solutions of ammonium paramolybdate react with acids. A concentrated solution has a pH of 5 to 6.
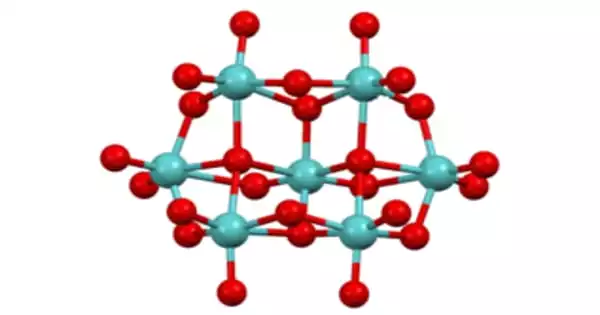
Structure
Lindqvist was the first to examine the molecule crystallographically, however it has since been reanalyzed. Mo centers are all octahedral. Some oxide ligands are terminal, whereas others are doubly bridging and a few are triply bridging.
Uses
It is a reagent used to measure phosphate, nickel, germanium, selenium dioxide, arsenate, alkaloid, lead, and other elements. Chromatographic analysis reagents Industrial as a catalyst for the oil sector, but also molybdenum powder metallurgy, photography, ceramic glaze, pigment industrial raw materials. It is also used as –
- as an analytical reagent to measure the amount of phosphates, silicates, arsenates, and lead in an aqueous solution (e.g. pigments, river water, seawater etc.)
- in the production of molybdenum metal and ceramics
- in the preparation of dehydrogenation and desulfurization catalysts
- in the fixing of metals and electroplating
- in fertilizers for crops.