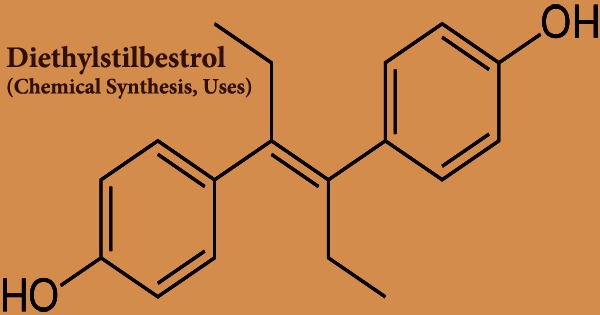
Diethylstilbestrol (DES), also called stilbestrol or stilboestrol, is a nonsteroidal estrogen that is synthesized. Diethylstilbestrol, a well-known teratogen, and carcinogen inhibits the hypothalamic-pituitary-gonadal axis, preventing testicular testosterone synthesis, reducing plasma testosterone, and producing chemical castration. It was once widely used for a variety of purposes, including pregnancy support for women who had previously miscarried, hormone therapy for menopausal symptoms and estrogen shortage in women, prostate cancer treatment in men and breast cancer treatment in women, and other uses.
Diethylstilbestrol is a stilbene derivative that varies from sin-estrol by having a double bond with the two phenyl groups trans-configured. It’s an agonist of the estrogen receptors, which are the biological targets of estrogens like estradiol. When compared to estradiol, DES has a far higher bioavailability when taken orally, is more resistant to metabolism, and has stronger effects in particular areas of the body, such as the liver and uterine. The Food and Drug Administration (FDA) issued a Drug Bulletin in 1971, warning doctors to stop providing DES to pregnant women because it has been associated to a rare form of vaginal cancer in female children.
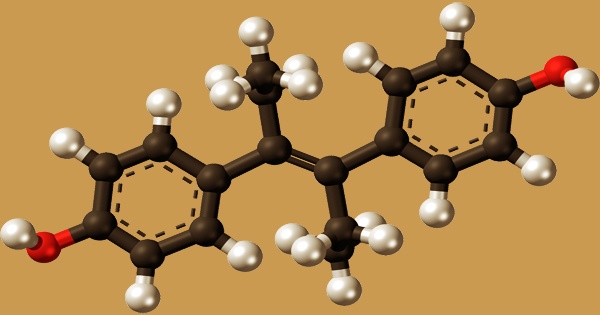
In 1938, DES was developed, and in 1939, it was approved for medicinal usage. It’s an olefinic molecule that’s trans-hex-3-ene with p-hydroxyphenyl groups replacing the hydrogens at positions 3 and 4. According to one of them, desoxyansoine is alkylated by ethyl iodide in the presence of sodium ethoxide, and the resultant ketone (28.1.30) is reacted with ethylmagnesium bromide in a Grignard reaction, resulting in the carbinol (28.1.31). The use of DES to treat prostate cancer in males is still a hot topic.
However, because of the lower risk of cardiovascular toxicity, bioidentical parenteral estrogens like polyestradiol phosphate have been recommended over oral synthetic estrogens like DES. In addition to prostate cancer, there is still some interest in using DES to treat breast cancer in women. It functions as an antineoplastic, carcinogenic, xenoestrogen, inhibitor of EC 3.6.3.10 (H(+)/K(+)-exchanging ATPase), antifungal, endocrine disruptor, an inhibitor of EC 1.1.1.146 (11beta-hydroxysteroid dehydrogenase), autophagy inducer, and calcium channel blocker.
DES is a polyphenol that also happens to be an olefinic molecule. Distillation in the presence of p-toluenesulfonic acid yields dimethyl ether of stilbestrol (28.1.32), whose methyl groups are removed by heating it at high temperatures with potassium hydroxide, resulting in diethylstilbestrol (28.1.33). DES is an odorless, tasteless white crystalline powder that releases unpleasant smoke and odors when heated to breakdown. In males undergoing androgen deprivation therapy for prostate cancer, oral DES at 0.25 to 0.5 mg/day is beneficial in the treatment of hot flashes.
DES has also been used to treat prostate cancer by inhibiting LH release from the pituitary, which reduces testicular androgen production. DES is linked to a significant rate of adverse effects, including nausea, vomiting, abdominal discomfort, headache, and bloating (incidence of 15–50%) at doses greater than 1 mg/day. Diethylstilbestrol and other stilbenes, on the other hand, are still available in many countries for the treatment of hormone-dependent neoplasms such as prostate cancer and postmenopausal breast cancer.
Following oral treatment, DES is quickly absorbed from the GI tract. The drug is slowly inactivated in the liver and eliminated primarily as the glucuronide in the urine and feces. DES has been linked to a high rate of gynecomastia (breast growth) in men treated for prostate cancer, ranging from 41 to 77%. Strong oxidizing agents, strong bases, acid chlorides, and acid anhydrides are incompatible with diethylstilbestrol. DES has been linked to significant cardiovascular morbidity and death in studies of the drug as a form of high-dose estrogen therapy for men with prostate cancer.
Diethylstilbestrol (DES) does not have a flashpoint; yet, it is most likely flammable. All three isotypes of the estrogen-related receptors (ERRs), the ERRα, ERRβ, and ERRγ, have been identified as antagonists of DES. At a concentration of around 1 μM, half-maximal inhibition occurs. The exact mechanism(s) of action of DES as a postcoital contraceptive is unknown; however, when given within 72 hours of coitus, the medicine appears to prevent nidation (implantation) of the fertilized ovum in the endometrium. Reduced quantities of circulating progesterone, effects on tubal motility resulting in the faster passage of the ovum into the uterus, and suppression of carbonic anhydrase production in the endometrium may all play a role in the drug’s postcoital contraceptive efficacy.
Ingestion can cause aberrant spermatogenesis, changes in the testes, epididymis, and sperm duct, changes in the menstrual cycle, changes in female ferulity, unexplained maternal effects, fetal urogenital system developmental abnormalities, germ cell effects in offspring, and delayed effects in newborns. The UK Medical Research Council (MRC) supported the DES study, which had a policy against patenting medications found with public monies. DES was developed by more than 200 pharmaceutical and chemical businesses throughout the world because it was not trademarked. Diethylstilbestrol in feeds is measured using thin-layer chromatography, which has a sensitivity of 0.07 mg/kg.
Information Sources: