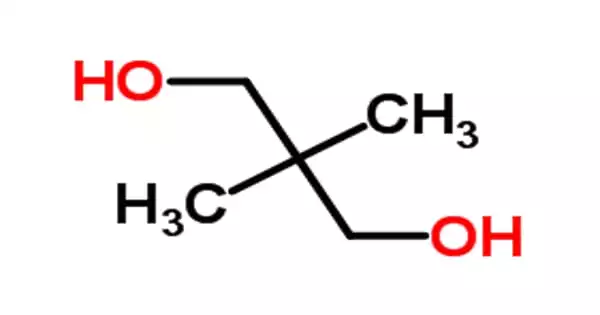
Neopentyl glycol is a chemical compound that is organic in nature. It is a colorless-to-white, hygroscopic crystalline, an organic chemical compound that is used in the synthesis of paints, polyesters, lubricants, and plasticizers. When used in the production of polyesters, it improves the product’s resistance to heat, light, and water.
Synthetic lubricating esters with lower oxidation or hydrolysis potential than natural esters can be produced via esterification reactions with fatty or carboxylic acids. If ingested or absorbed through skin contact, the combustible, slightly toxic compound can be harmful. It is known to irritate the skin and the eyes.
Properties
The melting point of neopentyl glycol is 1270 degrees Celsius. This solid flake form has a tendency to fuse together and form a block, making separation, transfer, and handling difficult. This functional glycol can be used as an intermediate in the production of plasticizers as well as synthetic lubricants. It is the sole glycol component in the majority of polyester resin formulations. It has exceptional thermal stability, weathering resistance, and stain resistance.
- Appearance: White Solid
- Boiling Point: 208 °C
- Density: 0.98 g/cm3
- Melting Point: 129.13 °C
- Molar Mass: 104.14 g/mol
- Solubility: 830 g/l
Plastic crystals of neopentyl glycol have been shown to have a colossal barocaloric effect (CBCEs), which is a cooling effect caused by pressure-induced phase transitions. Near room temperature, the obtained entropy changes are approximately 389 joules per kilogram per kelvin. This CBCE phenomenon will almost certainly be very useful in future solid-state refrigeration technologies.

Reactions
Industrially, neopentyl glycol is produced through the aldol reaction of formaldehyde and isobutyraldehyde. This produces the intermediate hydroxypivaldehyde, which can be converted to neopentyl glycol via a Cannizzaro reaction with excess formaldehyde or by palladium-on-carbon hydrogenation.
Industrially, the crystalline substance is produced by the aldol reaction of formaldehyde and isobutyraldehyde, which yields the intermediate hydroxypivaldehyde. Excess formaldehyde or catalytic hydrogenation of the aldehyde group to an alcohol group can be used to convert this to Neopentyl glycol.
Neopentyl glycol is soluble in water, benzene, chloroform, and ethanol, and it is very soluble in diethyl ether. When used in the production of polyesters, it improves the product’s resistance to heat, light, and water.
Application
It is used as a protecting group for ketones, such as in the synthesis of gestodene. Dyes, functional fluids in closed systems, intermediates, lubricants and lubricant additives, paint and coating additives, and processing aids specific to petroleum production are among the industrial applications for the combustible organic compound.
Adhesives and sealants, paints and coatings, and paper products are examples of consumer applications. It is the industry standard for high-performance coating resins. NPG’s polyesters synthesized with saturated or unsaturated di-acids are an excellent ingredient for paints, lubricants, plasticizers, and fiberglass-reinforced plastics.