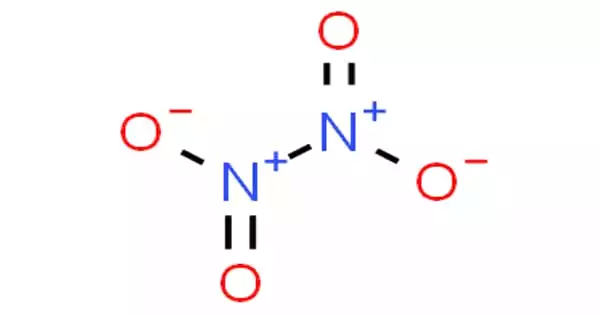
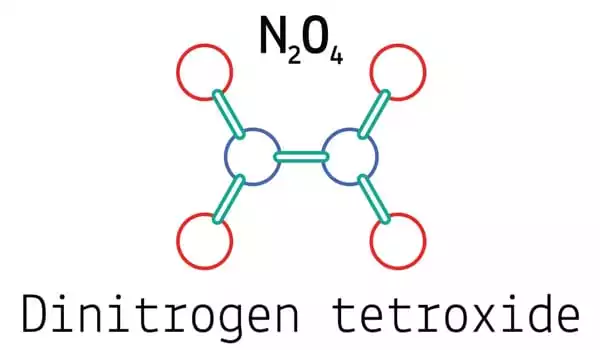
Dinitrogen tetroxide (N2O4) is a chemical molecule that is also known as nitrogen tetroxide (NTO) and amyl. It has the appearance of a red-brown liquid with a strong, unpleasant chemical odor. It is an important reagent in chemical synthesis. It combines with nitrogen dioxide to generate an equilibrium combination. It has a molecular mass of 92.011 g/mol.
Dinitrogen tetroxide is a potent oxidizer that is hypergolic (reacts spontaneously) when it comes into contact with various kinds of hydrazine, making the combination a popular rocket bipropellant.
Structure and properties
Dinitrogen tetroxide is made up of two nitro groups (-NO2) that are bound together. It combines with nitrogen dioxide to generate an equilibrium combination. The molecule is planar, with a 1.78 Å, N-N bond distance, and a 1.19 N-O distance. Because it is much longer than the usual N-N single bond length of 1.45 Å, the N-N distance corresponds to a weak bond. This unusually weak bond (amounting to the overlapping of the sp2 hybrid orbitals of the two NO2 units) is caused by the simultaneous delocalization of the bonding electron pair across the entire N2O4 molecule, as well as the significant electrostatic repulsion of the doubly occupied molecular orbitals of each NO2 unit.
N2O4 is diamagnetic, as opposed to NO2, since it contains no unpaired electrons. According to the following equilibrium, the liquid is also colorless but can seem reddish-yellow due to the presence of NO2:
N2O4 ⇌ 2 NO2
As the temperature rises, the equilibrium shifts toward nitrogen dioxide. Some dinitrogen tetroxide is unavoidably a component of nitrogen dioxide-containing smog.
Production
Nitrogen tetroxide is made by the catalytic oxidation of ammonia: steam is used as a diluent to reduce the combustion temperature. In the first step, the ammonia is oxidized into nitric oxide:
4 NH3 + 5 O2 → 4 NO + 6 H2O
Most of the water is condensed out, and the gases are further cooled; the nitric oxide that was produced is oxidized to nitrogen dioxide, which is then dimerized into nitrogen tetroxide:
2 NO + O2 → 2 NO2
2 NO2 ⇌ N2O4
and the remainder of the water is removed as nitric acid. The gas is essentially pure nitrogen dioxide, which is condensed into dinitrogen tetroxide in a brine-cooled liquefier.
Dinitrogen tetroxide can also be produced by reacting strong nitric acid with metallic copper. In a laboratory context, this synthesis is more practical, and it is widely used as a demonstration or experiment in college chemistry labs. The oxidation of copper by nitric acid is a complicated reaction that results in the formation of numerous nitrogen oxides with varying stability depending on the quantity of nitric acid, the presence of oxygen, and other conditions. The unstable species then react to produce nitrogen dioxide, which is purified and condensed to produce dinitrogen tetroxide.
Applications
It is used commercially as an intermediate in the manufacture of HNO3 and as a nitrating and oxidizing agent. N4O4 is the oxidizer of choice for storable rocket propellants because it is hypergolic with hydrazine fuels.