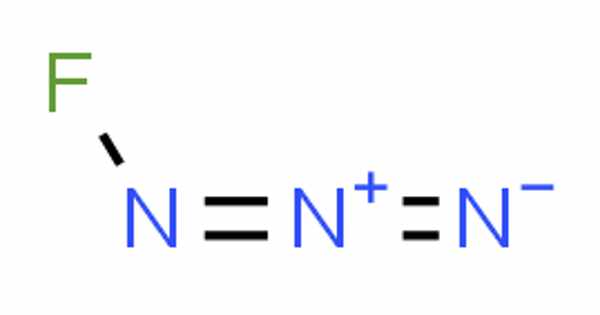Fluorine azide, also known as triazadienyl fluoride (FN3), is a yellow-green gas that is composed of nitrogen and fluorine and has the formula FN3. Because the azide functional group is a pseudohalogen, it is classified as an interhalogen compound. In this regard, it is similar to ClN3, BrN3, and IN3. The gas liquefies at –82° and can be solidified at –152°C. John F. Haller created it for the first time in 1942.
Properties
Because the bond between the fluorine atom and the nitrogen atom is very weak, this substance is very unstable and prone to explosion. According to calculations, the F–N–N angle is around 102° with a straight line of three nitrogen atoms.
The gas boils at –30° and melts at –139 °C.
It was first made by John F. Haller in 1942.
- Molecular Formula: FN3
- Molecular Weight: 61.01850
- Exact Mass: 61.00760
- Molecular Weight: 61.01850
- Density: N/A
- Boiling Point: N/A
- Melting Point: N/A
A molecule’s chemical structure includes the arrangement of atoms as well as the chemical bonds that hold the atoms together. There are three bonds in the fluorine azide molecule (s) There are three non-H bonds, two multiple bonds, one double bond, and one triple bond (s).
Reactions
Fluorine azide can be made by reacting to hydrazoic acid and fluorine gas. Another way to form it is by reacting sodium azide with fluorine.
Fluorine azide decomposes without explosion at normal temperatures to make dinitrogen difluoride: 2 FN3 → N2F2 + 2 N2.
At higher temperatures such as 1000 °C fluorine azide breaks up into nitrogen monofluoride radical: FN3 → FN{a1Δ} + N2.
The solid or liquid FN3 explodes, releasing a large amount of heat. A thin layer of film burns at a rate of 1.6 km/s. Because of the high risk of explosion, only very small amounts of this substance should be handled at a time. For experiments, a limit of 0.02 g is recommended.
N3F adducts can be formed with the Lewis acids boron trifluoride (BF3) and arsenic pentafluoride (AsF5) at -196°C. These molecules bond with the Nα atom.