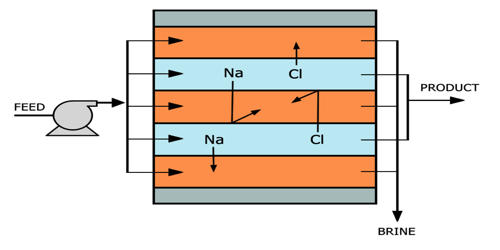
Electrodeionization (EDI) is an electrically-driven water treatment technology that uses electricity, ion exchange, and resin to remove ionized species from water. It uses no chemicals and generates no hazardous waste. It is a water treatment technology that utilizes electricity, ion exchange membranes, and resin to deionize water and separate dissolved ions (impurities) from water. High purity water production has traditionally used a combination of membrane separation and ion exchange processes. The combination of ion-exchange resins and ion-exchange membranes, which are used to move ionic impurities into a waste or concentrate water stream leaving purified product water. It is a continuous, chemical-free process of removing ionized and ionizable species from feedwater using DC power. Electrodeionization is designed to polish the permeate once the water has already gone through a water filtration system, such as reverse osmosis.
EDI is a process that combines semi-impermeable membrane technology with ion-exchange media to provide a high-efficiency demineralization process. It differs from other water purification technologies in that it is done without the use of chemical treatments and is usually a polishing treatment to reverse osmosis (RO). EDI is typically used to polish reverse osmosis (RO) permeate, and is a smart alternative to – and effective replacement of – conventional mixed bed ion exchange. There are also EDI units that are often referred to as continuous electro deionization (CEDI) since the electric current regenerates the resin mass continuously. CEDI technique can achieve very high purity, with a conductivity below 0.1 μS/cm. EDI has no hazardous wastewater discharge and wastes can be easily recycled without the neutralization systems needed for ion exchange. This system utilizes low energy consumption and disposes of the need for costly and unsafe chemicals used in conventional ion exchange water filters.
EDI operates continuously which minimizes the risk of breakthroughs from exhausted resin and simplifies operations by eliminating the need to take a system offline for regeneration. Recently, Argonne National Laboratory developed a process called Resin-Water Electrode ionization (RW-EDI), which uses a unique porous resin wafer mold made from immobilized loose ion-exchange resin beads. The resin wafer material enhances mass transfer between solid (resin bead) and liquid (feed solution) phases to achieve high purity, especially when treating impaired or brackish water.
EDI is used with reverse osmosis to replace ion-exchange resin-mixed beds, which require onsite or offsite chemical regeneration. Using electro deionization eliminates the need to store and handle the hazardous chemicals used for resin regeneration in mixed beds. The EDI process produces industrial process water of very high purity, using less than 95% of the chemical products used in the conventional ion exchange processes. Its development and use in water purification overcame some of the limitations of ion exchange resin beds, particularly the release of ions as the beds exhaust.