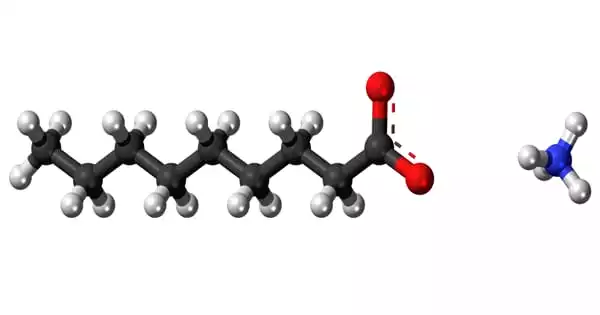
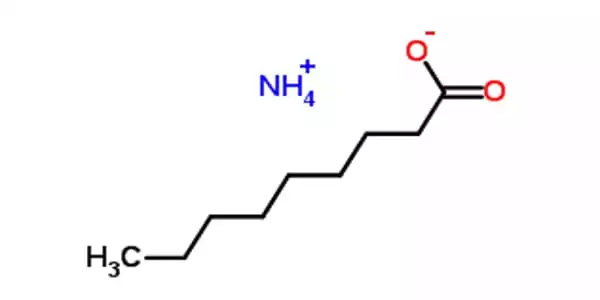
Ammonium Nonanoate is a nonanoic acid ammonium salt or “soap salt” (sometimes referred to as pelargonic acid). This fatty acid occurs naturally in practically all living creatures and is found in a wide variety of foods. It is made from a nonanoic acid, which is found in practically all plants and animals.
Ammonium nonanoate is a nonsystemic, broad-spectrum contact herbicide with no soil activity. It can be used to suppress and control weeds such as grasses, vines, underbrush, and annual/perennial plants such as moss, saplings, and tree suckers. Ammonium nonanoate is sold as an aqueous solution. At ambient temperature, at its highest concentration in water (40 percent). Solutions are colorless to pale yellow liquids with a faint fatty acid flavor. It is stable in storage. Ammonium nonanoate crystals are white.
Ammonium nonanoate is derived from ammonia and nonanoic acid, a carboxylic acid found in nature mostly as derivatives (esters) in foods such as apples, grapes, cheese, milk, grains, beans, oranges, and potatoes, as well as many other nonfood sources.
Applications
Ammonium nonanoate is a multi-purpose herbicide that has been used to control weeds, underbrush, and mosses since its approval in 2006. It is a naturally occurring fatty acid that can be found in a wide range of plants and animals. Because certain fatty acids are also used to manufacture domestic soap, it is referred to as a soap-salt.
Ammonium nonanoate has no effect on the plant tissue it comes into touch with. That is, it does not spread to other parts of the plant, hence thorough spray coverage of the entire weed is critical.
When ammonium nonanoate comes into touch with plant tissue, it breaks plant cell membranes and lowers pH, resulting in plant cell death/desiccation within minutes. It can be used alone, but it is especially effective when combined with other herbicides designed to inhibit weed regrowth.