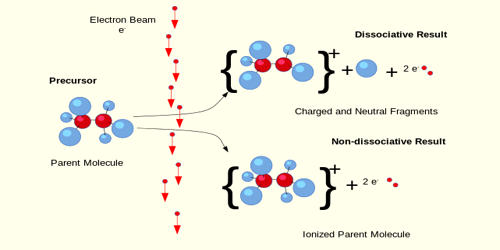
Electron ionization (EI) is an ionization method in which energetic electrons interact with solid or gas-phase atoms or molecules to produce ions. It is known as electron impact, which is an ionization method in which energetic electrons interact with gas-phase atoms or molecules to produce ions. EI was one of the first ionization techniques developed for mass spectrometry. However, this method is still a popular ionization technique. This technique was early introduced by Dempster in 1918 and is widely used for gases and volatile molecules. The EI source is generally composed of a cathode (filament), an ion chamber, an electron receiver, and a set of electrostatic lenses.
This technique is considered a hard (high fragmentation) ionization method since it uses highly energetic electrons to produce ions. This leads to extensive fragmentation, which can be helpful for the structure determination of unknown compounds. EI involves the interaction of a low-pressure (∼10−1 Pa) gas cloud with electrons that have been accelerated through a 70-V electric field. EI is the most useful for organic compounds which have a molecular weight below 600. EI is much suitable for the ionization of volatile lipid molecules. Also, several other thermally stable and volatile compounds in solid, liquid, and gas states can be detected with the use of this technique when coupled with various separation methods. This technique is considered to be a hard ionization method (high fragmentation) because it uses high-energy electrons to generate ions.
Applications
Electron ionization was the first ionization method developed for mass spectrometry, and it remains the most widely used. Since the early 20th-century electron ionization has been one of the most popular ionization techniques because of the large number of applications it has. It is the basis for one of the most efficient mass spectrometry methods for identifying a given organic compound. These applications can be broadly categorized by the method of sample insertion used. The ionization process is the direct result of the interaction of an energetic electron with the electrons in the molecule of interest. EI is useful for organic compounds with molecular weights below 600. The gaseous and highly volatile liquid samples use a vacuum manifold, solids and less volatile liquids use a direct insertion probe, and complex mixtures use gas chromatography or liquid chromatography. In addition, several other thermally stable and volatile compounds in solid, liquid, and gas states can be detected with the use of this technique when combined with various separation methods.
Advantages of EI
- Reproducible method
- High Ionization Efficiency
- Ionization is non-selective
- Interface to GC possible
- All vaporized molecules can be ionized (non-polar and insoluble)
- Molecular structural information.
Disadvantages of EI
- Only +ve ions are formed
- The sample has to be volatile
- Large internal energy method
- No interface to LC
- Limits to 600Da or less MW
- Limits value in MW determination due to extensive fragmentation.