Ammonium bicarbonate is an inorganic compound, also generally referred to as ammonium hydrogen carbonate. Due to its leavening and stabilizing properties and acid controlling ability, it is widely used in the bakery industry. In solution, ammonium bicarbonate is toxic and liberates ammonia and CO2. Its chemical formula (NH4)HCO3, simplified to NH5CO3; and the compound has many names, reflecting its long history. Chemically, it’s the salt of ammonium ion bicarbonate. It is a colorless solid that degrades carbon dioxide, ammonia, and water.
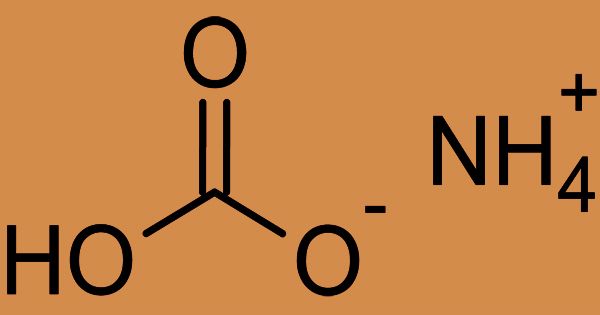
(2D model of Ammonium Bicarbonate)
The mixture of carbon dioxide and ammonia produces ammonium bicarbonate:
CO2 + NH3 + H2O → (NH4)HCO3
Ammonium bicarbonate is also used in MALDI mass spectrometric analysis of proteins for the in-gel digestion of proteins by trypsin. Since ammonium bicarbonate is thermally unstable, it holds the reaction solution cold, causing the substance to precipitate as a white solid. In 1997, about 100,000 tons were manufactured this way.
Ammonium bicarbonate, along with various other ammonium salts, is found in small quantities in nitrogenous organic matter. A solid aqueous sesquicarbonate solution (a 2:1:1 mixture of (NH4)HCO3, (NH4)2CO3, and H2O) transforms ammonia gas into natural ammonium carbonate ((NH4)2CO3), which can be obtained from a solution prepared at about 30oC in the crystalline state. This compound emits ammonia when exposed to air and reverts to ammonium bicarbonate. It is used in the processing of dyes, pharmaceuticals, catalysts, ceramics, fire-retardants, plastics, and other products in the industry.
Ammonium bicarbonate is used primarily as fertilizer. Popular applications of Ammonium bicarbonate:
- Cookies
- Crackers
- Pastas
- Frozen dairy products
- Bakery ingredients
- Pigments and paints
- Agriculture
- Fire extinguishers

(Uses of Ammonium Bicarbonate)
A leavening agent is a material that in baked goods releases gas to impart a light texture. This substance includes ammonium carbamate (NH4CO2NH2), and ammonium carbonate ((NH4)2CO3) in addition to ammonium bicarbonate. It is also called sesquicarbonate of ammonium.
It is simple to subject ammonium bicarbonate to decomposition. It is appropriate to pack with a mixture of inner plastic film and outer plastic bag or 3-layer kraft paper bags, all of which need to be sealed and stored in a cool, low-temperature, dry, and ventilated warehouse in order to escape moisture, rain, and sun. In nature, the compound occurs as an exceptionally rare teschemacherite mineral. The hazard to the atmosphere is the primary risk. In order to restrict distribution to the setting, urgent steps should be taken. After exposure to ammonium bicarbonate, health effects can occur at any time and may last for months or years.
Ammonium bicarbonate is a white crystalline solid that is soluble in water but insoluble in alcohol and acetone, with a slight ammonia odor. It breaks down into ammonia, carbon dioxide, and water vapor above 35°C, releasing irritant fumes. The most common additives used in the bakery industry as leavening agents are ammonium bicarbonate and sodium bicarbonate since they release carbon dioxide when certain temperature and humidity conditions are reached.
Information Sources: