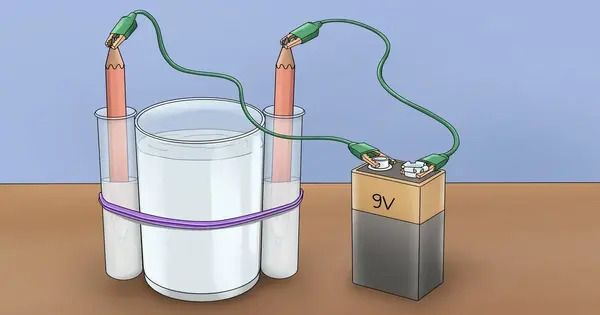
Water electrolysis is a chemical process that uses electricity to divide water molecules (H2O) into hydrogen (H2) and oxygen (O2) gases. Electrolysis is the process of splitting water into oxygen (O2) and hydrogen (H2) gas. Hydrogen gas produced in this manner can be utilized as hydrogen fuel, but it must be maintained separately from oxygen because the mixture would be exceedingly explosive.
Separately pressurized into suitable ‘tanks’ or ‘gas bottles’, hydrogen can be utilized for oxyhydrogen welding and other purposes, as the hydrogen/oxygen flame can reach temperatures of around 2,800 degrees. This process takes place in an electrolytic cell, which is normally made up of two electrodes (usually made of inert materials like platinum or graphite) immersed in a solution of water and an electrolyte like sulfuric acid or sodium hydroxide.
When an electric current is passed through the electrolyte solution, water molecules are dissociated into their constituent elements at the electrodes:
- At the cathode (negative electrode): 2H2O(l) + 2e- → H2(g) + 2OH-(aq)
- At the anode (positive electrode): 2H2O(l) → O2(g) + 4H+(aq) + 4e-
- Overall reaction: 2H2O(l) → 2H2(g) + O2(g)
The hydrogen gas collects at the cathode, while the oxygen gas collects at the anode. The gases can then be collected separately.
This technique is widely employed in a variety of commercial applications, including hydrogen synthesis for fuel cells, industrial operations that require hydrogen or oxygen, and gas creation in laboratories. Furthermore, it is one of the methods for producing hydrogen as a clean and renewable energy source, particularly if the electricity needed for electrolysis comes from renewable sources such as solar or wind power.
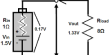
Water electrolysis requires a minimum potential difference of 1.23 volts, although even at that voltage, external heat is necessary. Typically, 1.5 volts are necessary. Electrolysis is rarely used in industrial applications since hydrogen can be produced more cheaply from fossil fuels.