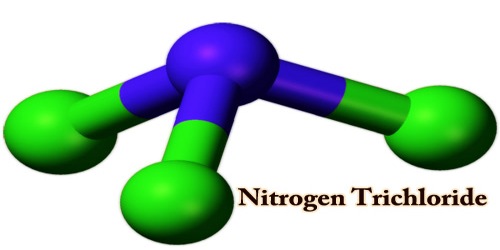
Nitrogen trichloride, also known as trichloramine, is prepared by the absorption of chlorine gas into a slightly acid ammonium chloride solution. That is the chemical component with the formula NCl3. This yellow, sticky, pungent-smelling, and explosive liquid is most commonly used as a by-product of chemical reactions between ammonia-derivatives and chlorine (in swimming pools for example). The substance is extracted permanently with carbon tetrachloride:
NH4Cl + 3Cl2 → NCl3 + 4HCl

In addition to monochloramine and dichloramine, trichloramine is responsible for the distinctive ‘chlorine smell’ associated with swimming pools, where the compound is readily formed as a result of hypochloric acid that interacts in the water with ammonia and other nitrogenic substances. In such preparation, hypochlorous acid, HOCl, can also be used in place of chlorine. The action of anhydrous chlorine with anhydrous ammonia will help prepare nitrogen trichloride:
3Cl2 + NH3 → NCl3 + 3HCl
NCl3 is a pyramidal molecule, like ammonia. The distances to N-Cl are 1.76 Å, and the angles to Cl-N-Cl are 107°. Nitrogen trichloride is commercially produced by electrolyzing ammonium chloride, an acidified solution. The chemistry of NCl3 was well investigated. It is moderately polar with a moment of 0.6 D in the dipole. The core of nitrogen is essential but much less so than ammonia. Hot water is hydrolyzed to absorb ammonia and hypochlorous acid.
Nitrogen trichloride (NCl3) explodes when heated to approximately 200F (93oC) or when exposed to direct sunlight. A high irritant; poisonous by ingestion and inhalation. NCl3 can be produced in small quantities when public water sources are disinfected with monochloramine, and in swimming pools by disinfecting urea-reacting chlorine in urine and bathers’ sweat. It’s soluble in benzene, chloroform, dissolving agent, chemical compound, and phosphorus trichloride.
Nitrogen trichloride, trademarked as Agene, was at just once accustomed bleach flour, but this practice was banned within the United States in 1949 because of safety concerns. An irritant to the eyes, skin, mucous membranes, and a systemic central system irritant. An explosive sensitive to impact, light, and ultrasound. Nitrogen trichloride is a lachrymatory agent that can irritate the mucous membranes but has never been used as such. The solid explodes when melted. The liquid is boiling above 60°C. There are also condensed solutions which are explosive.
Communication with: condensed ammonia, arsenic, dinitrogen tetraoxide, hydrogen sulfide, hydrogen trisulfide, nitrogen oxide, organic matter, ozone, phosphine, phosphorus, potassium cyanide, potassium hydroxide solutions, selenium, hydrogen chloride, hydrogen fluoride, hydrogen bromide, and hydrogen iodide starts explosive decomposition. Chlorine + hydrogen mixtures are potentially highly explosive. The pure (rarely encountered) material is a dangerous explosive which is sensitive to light, heat, even mild shock, and organic compounds.
Information Sources: