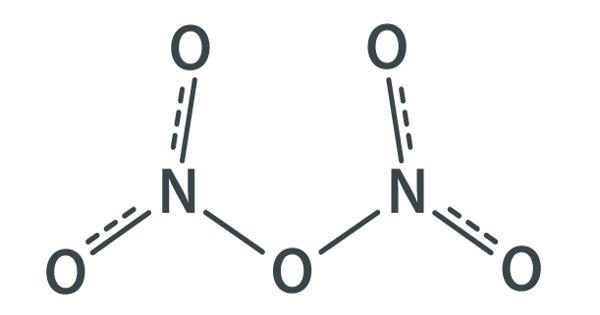
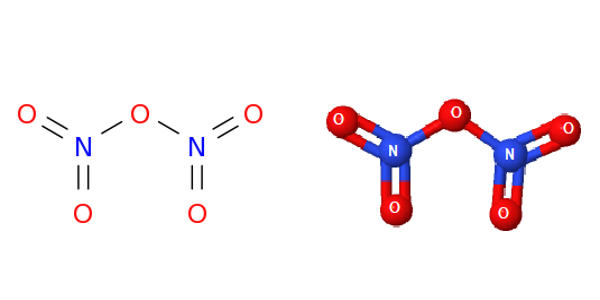
Dinitrogen pentaoxide is nitrogen oxide. It is the chemical compound with the formula N2O5, also known as nitrogen pentoxide or nitric anhydride. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It is the anhydride form of nitric acid, which can be made as white crystals, by carefully dehydrating nitric acid with diphosphorus pentoxide or by oxidizing nitrogen dioxide with ozone. It exists as colorless crystals that melt at 41°C. Its boiling point is 47°C, and sublimes slightly above room temperature, yielding a colorless gas. It is an unstable compound that decomposes spontaneously at room temperature into nitrogen dioxide and oxygen.
N2O5 is a rare example of a compound that adopts two structures depending on the conditions. It is a strong oxidizer and can form explosive mixtures with ammonium salts and organic compounds. When Nitrogen pentoxide decomposes it produces highly toxic nitrogen dioxide gas.
In nitric acid, to give a high concentration of nitronium cation, dinitrogen pentoxide is highly dissociated. The solid is a salt, nitronium nitrate, consisting of separate nitronium cations [NO2]+ and nitrate anions [NO3]–; but in the gas phase and under some other conditions it is a covalently bound molecule. Dinitrogen Pentoxide is an unstable and mildly explosive chemical of no current commercial value.
Properties
Its density is 1.642 gram per cubic cm. Its boiling point is 47-degree C and the melting point is 41 degrees C. It is heavier than air.
- Density: 1.64 g/cm³
- Molecular Weight/ Molar Mass: 108.01 g/mol
- Boiling Point: 47 °C
- Melting Point: 41 °C
- Appearance: White solid
- Solubility: Soluble in alcohol and ether
Preparation
A recommended laboratory synthesis entails dehydrating nitric acid (HNO3) with phosphorus(V) oxide:
P4O10 + 12 HNO3 → 4 H3PO4 + 6 N2O5
Another laboratory process is the reaction of lithium nitrate LiNO3 and bromine pentafluoride BrF5, in the ratio exceeding 3:1. The reaction first forms nitryl fluoride FNO2 that reacts further with the lithium nitrate:
BrF5 + 3LiNO3 → 3LiF + BrONO2 + O2 + 2FNO2
FNO2 + LiNO3 → LiF + N2O5
The compound can also be created in the gas phase by reacting nitrogen dioxide NO2 or N2O4 with ozone:
2NO2 + O3 → N2O5 + O2
However, the product catalyzes the rapid decomposition of ozone:
2O3 + N2O5 → 3O2 + N2O5
Dinitrogen pentoxide is also formed when a mixture of oxygen and nitrogen is passed through an electric discharge.
Atmospheric occurrence
In the atmosphere, dinitrogen pentoxide is an important reservoir of the NOx species that are responsible for ozone depletion: its formation provides a null cycle with which NO and NO2 are temporarily held in an unreactive state.
Uses
- It is used as a robust oxidizer in high-fuel rockets.
- It is used in solvents that are not based on water, so that molecules that are very sensitive to water can be easily nitrated.
- Used as a nitrating agent in modern synthetic organic chemistry.
Information Source: