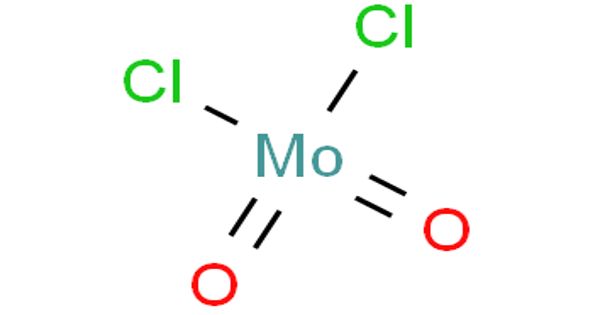
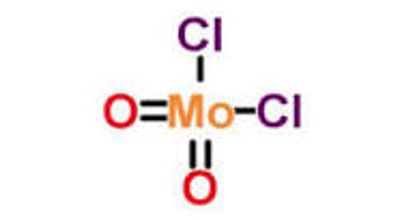
Molybdenum is much less toxic than typical heavy metals and already found application as an alternative substitute for some heavy metals. Molybdenum dichloride dioxide is the inorganic compound with the formula MoO2Cl2. It is a yellow solid that is used as a precursor to other molybdenum compounds. It is used to catalyze the oxidation of sulfides, thiols as well as the synthesis of beta-keto esters and thiocaetalization of aldehydes. It can also be used to analyze the antioxidant in solder plating solutions.
It is a nonmolecular solid but is often encountered as its adducts MoO2Cl2(ether)2, which are soluble in organic solvents. It is one of several oxychlorides of molybdenum. Molybdenum catalysts are resistant to poisoning by sulfur and hence used for conversion of hydrogen and carbon monoxide to alcohols even in the presence of sulfur which would poison precious metal catalysts.
Molybdenum dichloride dioxide is a yellow solid that is used as a precursor to other molybdenum compounds. It is used to catalyze the oxidation of sulfides, thiols as well as the synthesis of beta-keto esters and thiocaetalization of aldehydes.
Properties
- Metal Purity: 99.9%-Mo
- Form: solid (flakes)
- Appearance: yellow powder
- Melting point: 175°C
- Molecular Formula: MoO2Cl2
- Formula Weight: 198.84
Preparation
The compound is most easily prepared by treatment molybdenum trioxide with concentrated hydrochloric acid:
MoO3 + 2 HCl → MoO2Cl2 + H2O
MoO2Cl2 can also be prepared from MoOCl4:
MoOCl4 + O(Si(CH3)3)2 → MoO2Cl2 + 2 ClSi(CH3)3
It is also prepared by chlorination of molybdenum dioxide:
MoO2 + Cl2 → MoO2Cl2
It is prepared by reaction of MoO2 with dry chlorine. The reaction proceeds at 160°C. The crude product thus formed is purified by sublimation. Alternative preparation is to pass a 1:1 mixture of dry chlorine and oxygen over molybdenum metal at 250–350°C.
Uses
Molybdenum dichloride dioxide has a strong application as a kind of catalyst in organic transformation. It can be used to catalyze carbamate formation from alcohol and isocyanates, acylation reactions, thioglycosylation of functionalized peracetylated glycosides, hydrosilylation of aldehydes and ketones as well as reduction of many compounds like imines, esters, amides, and N-oxides.
Information Source: