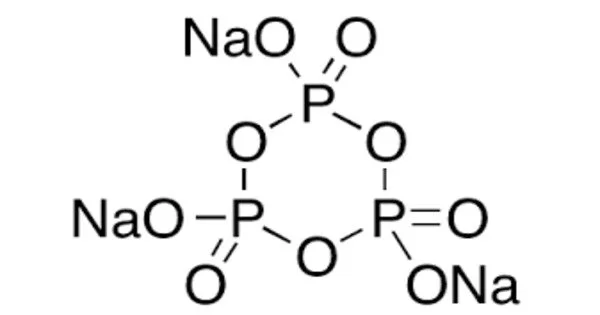
Sodium trimetaphosphate (also known as STMP) is a sodium metaphosphate with the formula Na3P3O9. It has the formula Na3P3O9, but it is also known as Na3P3O9•(H2O)6. Trimetaphosphoric acid is the sodium salt. It is a colorless solid with specialized applications in the food and building industries.
Despite being drawn with a specific resonance structure, the trianion has a high degree of symmetry. It is a sodium salt of trimetaphosphoric acid. It is an inorganic compound that is commonly used as a food additive, a water softener, and a dispersant in various industrial applications.
Properties
Sodium trimetaphosphate is a white crystalline powder that is soluble in water. STMP is often used as a sequestering agent in water treatment processes to prevent the precipitation of metal ions and to improve water quality. It can also be used as a dispersant in ceramics and as a builder in detergents to improve their cleaning performance.
- Chemical formula: Na3P3O9
- Molar mass: 305.885 g/mol
- Appearance: colorless or white crystals
- Density: 2.49 g/cm3 (anhydrous); 1.786 g/cm3 (hexahydrate)
- Melting point: 53 °C (127 °F; 326 K) (hexahydrate, decomposes to anyhdrous)
- Solubility in water: 22 g/100 mL
- Solubility: insoluble in alcohol
- Crystal structure: triclinic (hexahydrate)

Synthesis and reactions
Trisodium trimetaphosphate is produced industrially by heating sodium dihydrogen phosphate to 550 °C, a method first developed in 1955:
3 NaH2PO4 → Na3P3O9 + 3 H2O
The trimetaphosphate dissolves in water and is precipitated by the addition of sodium chloride (common ion effect), affording the hexahydrate. STMP can also prepared by heating samples of sodium polyphosphate, or by a thermal reaction of orthophosphoric acid and sodium chloride at 600°C.
3 NaH3PO4 + 3 NaCl → Na3P3O9 + 3 H2O + 3 HCl
Hydrolysis of the ring leads to the acyclic sodium triphosphate:
Na3P3O9 + H2O → H2Na3P3O10
The analogous reaction of the metatriphosphate anion involves ring-opening by amine nucleophiles.
Application
In the food industry, sodium trimetaphosphate is often used as a sequestrant and emulsifier. It helps to improve the texture and shelf life of processed foods, such as canned meats, cheeses, and baked goods. It is also used as a water softener in detergents and as a dispersant in paints, ceramics, and other industrial products.
STMP is often used as a sequestering agent in water treatment processes to prevent the precipitation of metal ions and to improve water quality. It can also be used as a dispersant in ceramics and as a builder in detergents to improve their cleaning performance.
Safety
It is generally considered safe for use in food and other applications, although it can be irritating to the skin and eyes in its pure form. As with any chemical, proper handling and safety precautions should be taken when working with sodium trimetaphosphate.
However, it is important to note that STMP is a phosphate compound, and excessive use or release of phosphates into the environment can contribute to eutrophication, a process in which bodies of water become overloaded with nutrients, leading to excessive algae growth and other negative impacts on aquatic ecosystems. As a result, many countries have regulations in place to limit the use and release of phosphates in various applications.