Purifying biopharmaceutical medications is still a time-consuming and expensive operation. Researchers may be able to make purification methods more efficient, more complicated, and scalable if they have a better grasp of how undesirable components in biomanufactured proteins interact to the compounds produced to remove them.
Process chromatography is expensive and uses a lot of solvents, thus it needs to be processed further downstream. Furthermore, chromatography is unsuitable for large-scale and continuous processes. As a result, creating novel continuous downstream processing processes is an appealing research and development topic.
A team led by Steven Cramer, an endowed chair professor of chemical and biological engineering at Rensselaer Polytechnic Institute, investigated the foundations of how different molecules interact with different surfaces during the purification process in a study published in Langmuir.
Cramer is a recognized expert in chromatographic bioprocessing, a separation technique used in biopharmaceutical purification to pick which components of a protein combination should be preserved and which should be discarded.
Ligands are ions or molecules that are designed to bind to specific components that should be kept or eliminated during the procedure. Purification is an important stage in the drug production process because it helps to reduce the amount of undesired components that can be harmful or damage drug efficacy.
“This is part of a very big effort to understand the fundamentals of how these molecules interact with surfaces,” said Cramer, a member of the Rensselaer Center for Biotechnology and Interdisciplinary Studies (CBIS), where the work was performed. “Our group is trying to ramp up the intellectual level of this kind of analysis in a variety of ways.”
This is part of a very big effort to understand the fundamentals of how these molecules interact with surfaces. Our group is trying to ramp up the intellectual level of this kind of analysis in a variety of ways.
Steven Cramer
This new work builds on studies published previously in Biotechnology and Bioengineering by the Cramer Lab.
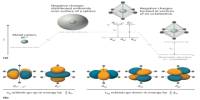
Researchers used nuclear magnetic resonance (NMR) spectroscopy and complex computer simulations to investigate the fundamentals of how different molecules interact with different surfaces and ligands, such as how and where binding occurs, and whether certain molecular interactions affect the binding process.
The researchers looked at the Fc component of an IgG1 antibody and how ligands interact with that section of the antibody protein in particular, working with Merck Pharmaceuticals and Bio-Rad Laboratories and using the NMR core facility in CBIS run by co-author Scott McCallum. (If you were to picture an antibody looking like the letter “Y,” the Fc part would be the tail.)
Because IgG1 antibodies are employed in so many biopharmaceutical treatments, a better knowledge of how their components interact with different compounds and ligands could have far-reaching effects.
The scientists went even further in the Langmuir publication, putting nanoparticles on the surface of several proteins to see exactly where the ligands were binding.
“With this approach, we can then also look at some of the subtle interactions that are happening,” Cramer said. “Sometimes these ligands come together and form these clusters of ligands, and these clusters actually drastically change the behavior.”
According to Cramer, this research could lead to the creation of new and superior materials and ligands. It could also aid researchers in developing more sophisticated and specific methods for filtering out undesired molecules that are very similar to molecules that must remain.
All of these developments have the potential to make the medication purification process more efficient and effective.