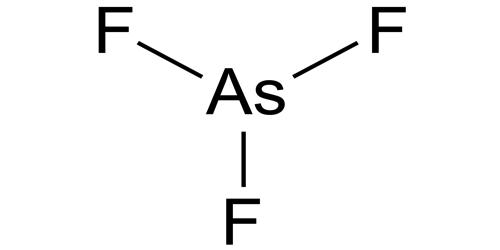
Arsenic is a semimetal (metalloid) that is capable of forming several compounds. Arsenic trifluoride is a chemical compound of arsenic and fluorine with the chemical formula AsF3. It is a colorless liquid which reacts readily with water. The common aromatic and alkyl derivatives of arsenic trichloride compounds are diphenylaminearsine (DM/adamsite), diphenylchlorarsine (DA), diphenylcyanoarsine (DC), ethyldichloroarsine, methyldichloroarsine, and phenyldichloroarsine. It is toxic if inhaled, is very toxic to aquatic life, is very toxic to aquatic life with long-lasting effects, causes severe skin burns and eye damage, and may cause cancer.
Properties
- Compound Formula: F3As
- Molecular Weight: 131.917
- Appearance: Colorless liquid
- Melting Point: -8.5 °C
- Boiling Point: 63 °C (760 mmHg)
- Density: 2.67 g/mL
- Solubility in H2O: Decomposes
- Exact Mass: 131.916804 g/mol
- Monoisotopic Mass: 131.916804 g/mol
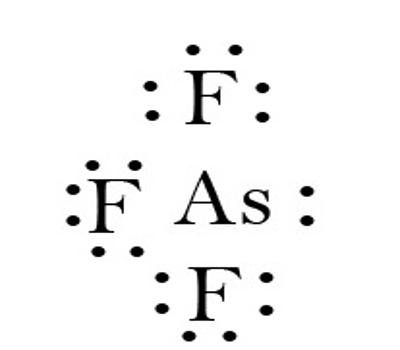
Fig: Chemical Bonding of Arsenic Trifluoride
Preparation and properties
It can be prepared by reacting hydrogen fluoride, HF, with arsenic trioxide:
6HF + As2O3 → 2AsF3 + 3H2O
It has a pyramidal molecular structure in the gas phase which is also present in the solid. In the gas phase, the As-F bond length is 170.6 pm, and the F-As-F bond angle 96.2°.
Arsenic trifluoride is used as fluorinating non-metal chlorides to fluorides, in this respect, it is less reactive than SbF3.
Salts containing AsF4− anion can be prepared for example CsAsF4. the potassium salt KAs2F7 prepared from KF and AsF3 contains AsF4− and AsF3 molecules with evidence of interaction between the AsF3 molecule and the anion.
AsF3 reacts with SbF5. The product obtained could be described as an ionic adduct of AsF2+ SbF6−. However, the authors conclude the formed product cannot be viewed only as an ionic compound nor entirely as a neutral adduct of AsF3SbF5. The crystal structure of the formed compound displays characteristics of both an ionic pair and neutral adduct structural motives, taking the middle ground in between both models of molecule description. Although acute intoxication has become rare, arsenic is still a dangerous pollution agent for industrial workers and people living in the vicinity of emission sources.
Safety
Contact with the eyes causes severe irritation or burns. Burns are progressive while any unneutralized fluoride ions remain. Contact with skin causes severe irritation or burns. Certain inorganic arsenic compounds have been associated
with a carcinogenic response to the skin in animals and humans. Although acute intoxication has become rare, arsenic is still a dangerous pollution agent for industrial workers and people living in the vicinity of emission sources.