Phosphorus is one of the most abundant elements on the planet. Phosphate salts may be found in virtually all igneous rocks, as well as sedimentary deposits and marine beds. Red phosphorus is a derivative of the P4 molecule and is one of the most frequent allotropes of phosphorus. It may be made by heating white phosphorus to 300 degrees Celsius (572 degrees Fahrenheit) in the absence of air or by exposing it to sunlight.
Phosphorus is found in about 300 minerals and is commonly found with Ca, Mg, Fe, Sr, Al, Na, and other metals, as well as anions such silicates, sulfates, oxides, hydroxides, and halides. The amorphous red phosphorus network exists as an amorphous network that crystallizes when heated further. It does not ignite in air at temperatures below 240 degrees Celsius (464 degrees Fahrenheit), whereas white phosphorus fragments ignite at around 30 degrees Celsius (86 degrees Fahrenheit). Phosphorus is a necessary component of all living things and plays an important role in biological and ecological processes.
The bright red color and granular texture of red phosphorus distinguish it. Pyrotechnics, smoke bombs, incendiary shells, and safety matches all include phosphorus. It’s also utilized in organic synthesis, as well as the production of phosphoric acid, phosphorus trichloride, phosphine, and other chemicals. The red phosphorus allotrope is formed by gradually transforming white phosphorus. In the presence of light and energy in the form of heat, this transformation occurs more quickly.
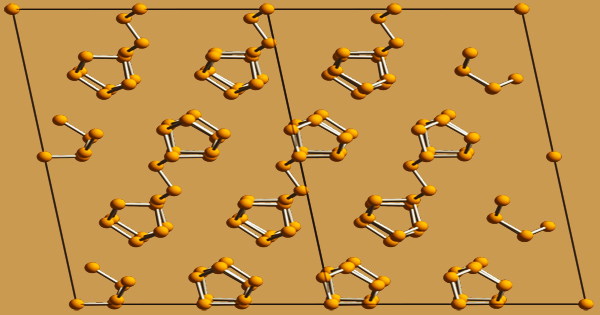
When white phosphorus is partly transformed to red phosphorus, it takes on a distinctive yellow color. With finely split material, ignition occurs spontaneously at room temperature because the large surface area allows surface oxidation to rapidly heat the sample to the ignition point. Red phosphorus is found in a polymeric chain of tetrahedrally structured P4 molecules, one of which has one of its P-P bonds broken to allow the tetrahedrons to be linked.
In the solid-state, there are three primary allotropic forms of phosphorus: (1) white or yellow phosphorus with alpha or beta modification, (2) red phosphorus, and (3) black phosphorus. Anton von Schrotter, an Austrian scientist, developed red phosphorus in 1845. He created red phosphorus by heating white phosphorous to 482oF in the presence of nitrogen for a few hours. It is more stable than white phosphorus under normal circumstances, but less stable than thermodynamically stable black phosphorus.
Red phosphorus has a standard enthalpy of production of -17.6 kJ/mol and is the kinetically most stable. It is made from white phosphorus by heating it to 230 to 240°C for around 48 hours, allowing for full conversion. Sulfur, iodine, and selenium catalyze the conversion. Red phosphorus crystallizes when heated to temperatures above 300oC, and its crystal lattice can also take on a cubic shape.
Red phosphorus undergoes a number of transformations. At ordinary temperatures, an amorphous form exists, as well as two crystalline modifications: a triclinic form and a hexagonal or tetragonal form that may exist at higher temperatures. The chemical reactivity of red phosphorus is lower than that of white phosphorus. At ordinary temperatures, the triclinic variation of red phosphorus is the most stable of all phosphorus allotropes.
The red phosphorus is made by heating white phosphorus (which must be immersed in water) to 550 degrees Fahrenheit in a steel kettle for 3-4 days. Matches include red phosphorus, which is quite safe. In high-strength low-alloy steel, ferrophosphorus, a mixture of phosphorus and iron, is utilized as a component. Red phosphorus is significantly less hazardous than its white allotrope, but its vapors are quite unpleasant when burnt.
Red phosphorus is an excellent flame retardant, particularly in thermoplastics (such as polyamide) and thermosets (e.g. epoxy resins or polyurethanes). With the aid of a system that condenses the reflux, phosphorus waste in the form of vapour is avoided. To distill out the leftover white phosphorous, the temperature is raised to 673K after the 2-day mark. Stabilization and micro-encapsulation can significantly reduce the safety hazards related with phosphine production and red phosphorus friction sensitivity.
All animal bones and teeth, as well as most living tissue, contain phosphorus. Along with manganese nodules, phosphorus nodules may be discovered on the ocean floor. Red phosphorus is frequently utilized in various carrier systems as dispersions or master batches for simpler handling. This allotrope of phosphorus is utilized in many flares used as emergency signals to aid in the igniting process. This allotrope is also used to keep the flare burning for a longer period of time.
Phosphate-rich materials are burned in electric furnaces to drive out the phosphorus as a gas, which is subsequently condensed underwater. Sulfuric acid is used in another method to eliminate phosphorus. Red phosphorus, when combined with magnesium and a binder, may be used as a smoke device to quickly generate a smokescreen. It’s also utilized in methamphetamine manufacturing (commonly known as meth).
Red phosphorus in epoxy molding compounds causes increased leakage current in semiconductor devices, which is a long-standing issue. It has a lower level of reactivity than the white variant. It is not toxic, but high quantities can cause an explosion. Many thermoplastics and thermosetting plastics include red phosphorus as a flame retardant. It may be used to make hydrogen from water as an elemental photocatalyst. It’s also utilized in smoke bombs because of its stable but reactive nature.
Information Sources: