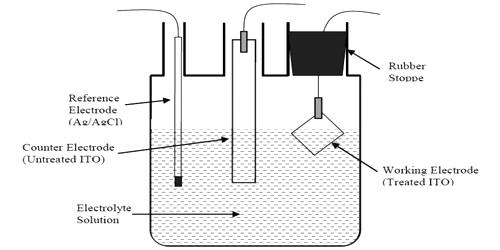
The working electrode (WE) represents the most important component of an electrochemical cell. It is probably the most important component of an electrochemical cell. It is the electrode in an electrochemical system on which the reaction of interest is occurring. The working electrode is a key factor in the process, directing the course of the electrochemical reaction according to its properties: material, adsorbent surface, etc. The working electrode is often used in conjunction with an auxiliary electrode, and a reference electrode in a three-electrode system. In corrosion testing, the working electrode is a sample of the corroding metal. Generally, it is not the actual metal structure being studied. Depending on whether the reaction on the electrode is a reduction or oxidation, the working electrode is called cathodic or anodic, respectively.
Common working electrodes can consist of materials ranging from inert metals such as gold, silver or platinum, to inert carbon such as glassy carbon, boron-doped diamond or pyrolytic carbon, and mercury drop and film electrodes. The working electrode is where the electrically driven chemical reaction and electron transfer happens. Several important factors should be considered. Firstly, the material should exhibit favorable redox behavior with the analyte, ideally fast, reproducible electron transfer without electrode fouling. Secondly, the potential window over which the electrode performs in a given electrolyte solution should be as wide as possible to allow for the greatest degree of analyte characterization. Being electrochemically inert and easy to be fabricated into many forms, platinum is often preferred. Chemically modified electrodes are employed for the analysis of both organic and inorganic samples. Metals such as platinum, gold, silver, nickel, mercury, gold amalgam, and a variety of alloys are now also commonly used as working electrode materials.
Working Electrode is also called an indicator electrode, it is used to reveal the properties of the analyte solution. If the working electrode is driven to a positive potential, relative to the reference electrode, an oxidation reaction can occur at the working electrode of the electrochemical cell. The most commonly used working electrode materials are platinum, gold, carbon, and mercury. Among these, platinum is likely the favorite, demonstrating good electrochemical inertness and ease of fabrication into many forms. A wide variety of working electrodes are available for use. The most common working electrode materials utilize carbon. Common working electrodes can consist of materials ranging from inert metals such as gold, silver or platinum, to inert carbon such as glassy carbon, boron-doped diamond or pyrolytic carbon, and mercury drop and film electrodes.