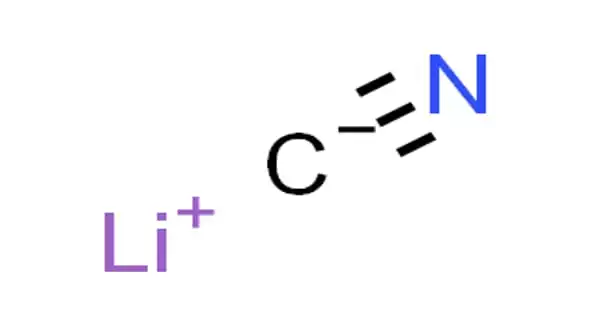Lithium cyanide, also known as LiCN, is an inorganic compound with the chemical formula LiCN. It’s a salt created by the reaction of the weak acid hydrocyanic acid, HCN, and the strong base lithium hydroxide. It is a toxic, white-colored, hygroscopic, water-soluble salt with only a few applications.
Cyanide is a toxic substance, primarily because of its affinity for the terminal cytochrome oxidase in the mitochondrial respiratory pathway, which reduces tissue oxygen utilization.
Properties
- Molecular Formula: CLiN
- Formula Weight: 32.96
- Melting point: 160℃ [KIR78]
- Density: 1.075
Preparation
LiCN arises from the interaction of lithium hydroxide and hydrogen cyanide. A laboratory-scale preparation uses acetone cyanohydrin as a surrogate for HCN:
(CH3)2C(OH)CN + LiH → (CH3)2CO + LiCN + H2
Hydrogen cyanide is created when certain materials are burned or pyrolyzed in an oxygen-deficient environment. It can be detected in the exhaust of internal combustion engines and tobacco smoke, for example. When heated or burned, certain plastics, particularly those derived from acrylonitrile, emit hydrogen cyanide.
In water, cyanide is unstable, but the reaction is slow until around 170 °C. It is hydrolyzed to produce ammonia and formate, both of which are far less toxic than cyanide:
CN– + 2 H2O → HCO2– + NH3
cyanide hydrolase is an enzyme that catalyzes this reaction.
Uses
The compound decomposes to cyanamide and carbon when heated to a temperature close to but below 600°C. Acids react to give hydrogen cyanide.
Lithium cyanide can be used as a reagent for organic compound cyanation.
RX + LiCN → RCN
It is commonly used in industry, particularly for metal cleaning and electroplating. It is a highly toxic species to living organisms (for example, bacteria used in sewage treatment can die), so when sewage concentrations are high, a previous pretreatment oxidation step must be performed before the effluent is discharged in an STP.
Toxicity
Lithium cyanide is rapidly absorbed from the skin and all mucosal surfaces; it is especially dangerous when inhaled because toxic amounts are rapidly absorbed through the bronchial mucosa and alveoli.
It is easily absorbed via inhalation, skin, and mucous membranes. The effects of ingestion are often delayed due to gastrointestinal absorption, whereas inhalation causes the most rapid onset of toxicity.