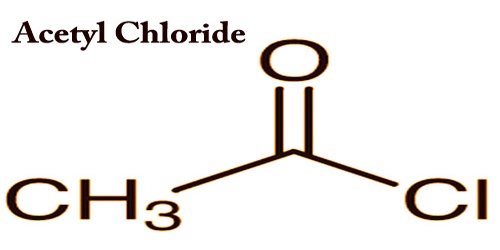
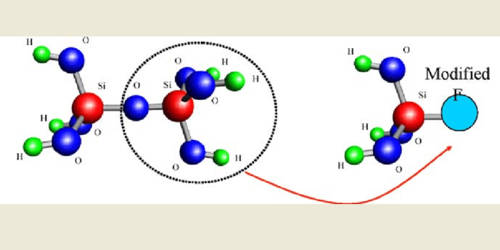
Acetyl chloride (CH3COCl) acts as a colorless fuming liquid with a powerful effect on odor and eye irritation. It is an acyl chloride derived from acetic acid; it belongs to the category of organic compounds called acyl halides. The density of 1.1051 (20 ° C), the freezing point of-112 ° C, the boiling point of 51-52 ° C and therefore the flashpoint is 4.4 ° C (closed dish). The heavier-than-air vapor irritates the eyes and mucous membranes. To metals and tissue corrosive.
Acetyl chloride can be violently hydrolyzed into acetic acid and hydrogen chloride as it comes across the water, and thus should be sealed for storage. On an industrial scale, the reaction of acetic anhydride with hydrogen chloride produces a mixture of acetyl chloride and acetic acid:
(CH3CO)2O + HCl → CH3COCl + CH3CO2H
Benzene, acetone, chloroform, alcohol, glacial acetic acid, and natural alcohol are miscible to acetyl chloride. Oil is heavier than water, since the vapor is heavier than air. The chemical character is lively. To generate esters and amides, it’s an effective acetylating agent for alcohols and amines. It is important in paint and pharmaceutical synthesis.
Acetyl chloride was first prepared by the French chemist Charles Gerhardt in 1852 when phosphoryl chloride was used to treat potassium acetate. It is used for yielding acetophenone in the Friedel-Craft acylation of benzene. This is commonly used in organic synthesis and is also the catalyst for carboxylic acid chlorination. It is produced by mixing glacial acetic acid and phosphorus trichloride in cold conditions with further heating, followed by distillation of the dihydrochloride.
Acetyl chloride can bind in the protein to the thiol group, and thus be toxic to humans. It is an essential form of acetylation agents, the potential for acylation being greater than acetic anhydride. Acetyl chloride is produced in the laboratory by the reaction of acetic acid with chlorodehydrating agents such as PCl3, PCl5, SO2Cl2, phosgene, or SOCl2.
Acetyl chloride may be a powerful acetylating agent, and it’s utilized in the manufacture of aspirin, acetaminophen, acetanilide, and acetophenone. Liquid compositions for optical display and memory devices frequently require acetyl chloride. Acetyl chloride could be quite stimulant and corrosive with contact with the skin having the ability to cause burns. Its steam can strongly irritate the attention and mucous membranes. The presence of acetyl chloride in nature is not expected, as contact with water would hydrolyze it into acetic acid and hydrogen chloride. Acetic chloride causes irreversible eye damage and serious burns to the mouth and stomach after contact with the eyes and skin, including accidental ingestion. A second major class of acetylation reactions is the Friedel-Crafts reactions.
Information Sources: