The type of electrolyte used in fuel cells is what distinguishes them. This classification defines the type of electrochemical processes that occur in the cell, the type of catalysts needed, the temperature range in which the cell functions, the fuel required, and other criteria. These properties, in turn, influence the applications for which these cells are most suited. There are various varieties of fuel cells in research right now, each with its own set of advantages, limitations, and potential applications.
A new high-temperature polymer fuel cell that operates at 80-160 degrees Celsius and has a higher rated power density than current fuel cells solves the long-standing problem of overheating, which is one of the most significant technical barriers to using medium- and heavy-duty fuel cells in transportation vehicles like trucks and buses.
Because contemporary fuel cells operate at temperatures ranging from 60 to 80 degrees Celsius, huge radiators and air intakes are required to keep them cold enough to operate. To address this issue, scientists at Los Alamos National Laboratory created a novel polymer fuel cell that can run at greater temperatures.
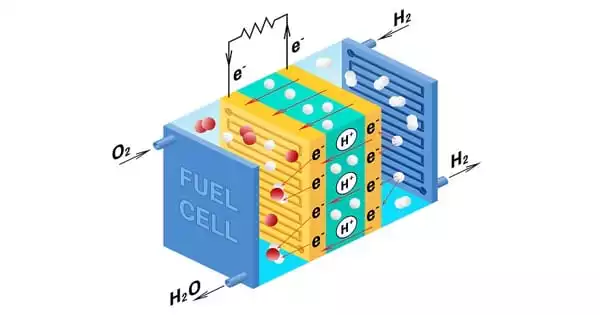
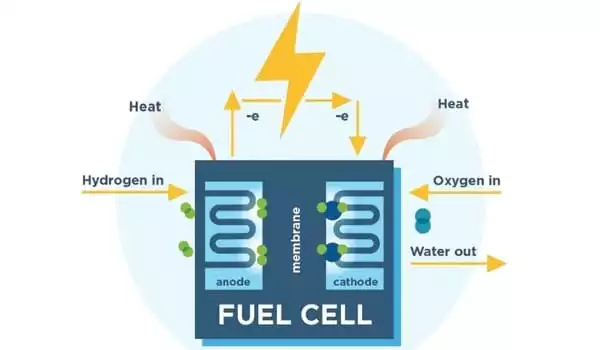
Fuel cells are energy conversion devices that generate electricity by electrochemically mixing hydrogen and oxygen from the surrounding environment. Fuel cell vehicles, like other electric vehicles, are zero-emission vehicles, with no smog-related or greenhouse gas tailpipe emissions.
Yu Seung Kim
“Fuel cells are energy conversion devices that generate electricity by electrochemically mixing hydrogen and oxygen from the surrounding environment. Fuel cell vehicles, like other electric vehicles, are zero-emission vehicles, with no smog-related or greenhouse gas tailpipe emissions” explained Yu Seung Kim of the Materials Synthesis & Integrated Devices division at Los Alamos. “Hydrogen can also be created from a variety of domestic resources, with the possibility for zero greenhouse gas emissions.”
Hydrogen fuel cells are an excellent choice for medium- and heavy-duty on-road transportation, such as trucks and buses, and they also have maritime, rail, and aviation applications.
Given global efforts to reduce transportation emissions, the electrification of future medium- and heavy-duty transportation – whether with batteries or hydrogen fuel cells – is necessary. Fuel cells, as opposed to battery-powered cars, provide quick fueling and ample fuel storage for long-range applications. Over several decades, researchers have investigated fuel cells that can run at temperatures beyond 100 degrees Celsius, allowing for simpler fuel cell systems through improved heat and water management. While more research is needed to verify the durability required for heavy-duty applications, this study gives a way for fabricating highly performing fuel cells in hot and dry circumstances.
Advances in fuel cell technology also help the Intermountain West Energy Sustainability & Transitions (I-WEST) effort, which is creating a technical roadmap to convert the western United States to a carbon-neutral, economically sustainable energy system. The roadmap will detail how the states of the Intermountain West can handle difficulties, capitalize on opportunities, and develop an equitable energy transition strategy.
Fuel cells are increasingly recognized as a dependable green energy alternative to polluting combustion or incineration processes, ranging from diesel and gasoline engines to coal-burning power plants. Rather than using a fuel to generate energy, fuel cells use an electrochemical reaction, which produces no greenhouse gases.
How they work
Phosphoric acid is used as an electrolyte at the electrode in conventional high-temperature polymer electrolyte membrane fuel cells. The Los Alamos researchers created a polymer electrolyte out of a phosphonated polymer and a perfluorosulfonic acid in this study. The scientists discovered that a proton from the perfluorosulfonic acid passes to the phosphonated polymer and greatly increases proton conductivity in this composite electrolyte structure. The researchers were able to attain a nearly 800 milliwatts per square centimeter rated power density of the fuel cell at 160 C by adopting the composite polymer electrolyte, which is a 60% improvement over the phosphoric acid-based fuel cells.
Hydrogen fuel cell technology is currently being extensively investigated around the world in order to create a dependable sustainable energy source. Global warming, natural disasters, and the scarcity of fossil fuels have focused global attention on environmentally friendly and renewable energy sources. The hydrogen fuel cell technology most emphatically meets those requirements. New research helps to improve fuel cell performance, endurance, cost-efficiency, and overcome restrictions. The numerous aspects influencing the characteristics and efficiency of a fuel cell must be investigated in a systematic manner during the development process. Temperature is a significant performance-changing parameter in Proton Exchange Membrane Fuel Cells (PEMFC).