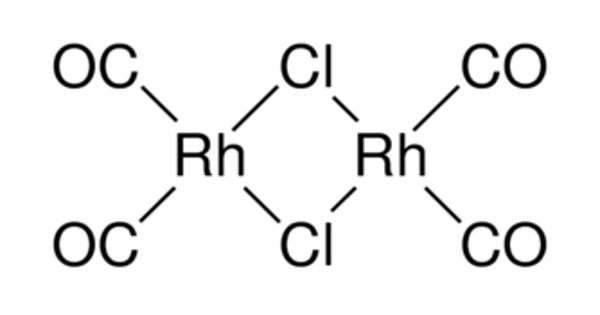
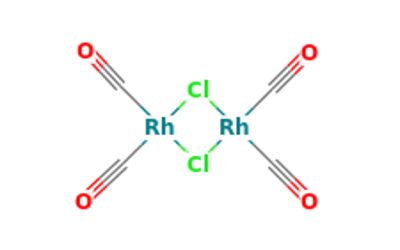
Rhodium carbonyl chloride is an organorhodium compound with the formula Rh2Cl2(CO)4. It is a red-brown volatile solid that is soluble in nonpolar organic solvents. It is a precursor to other rhodium carbonyl complexes, some of which are useful in homogeneous catalysis.
From rhodium carbonyl chloride, a series of novel bridged compounds, [Rh(C0)2X] (X = acetate, benzoate, nitrate.thiocyanate, sulphate, etc.), are prepared. Mononuclear-complex is from these bridged compounds, by-reaction- with amines and phosphines. A kind of method of preparing rhodium complex catalyst from reaction waste solution of olefin carbonylation rhodium catalyst.
The higher oxidation states of rhodium are, as expected, formed with the group 6 and 7 elements, fluorine in particular. Rhodium hexafluoride has been prepared in the gas phase, at elevated temperatures, by the action of fluorine on the metal, at room temperature, it decomposes to a lower fluoride and fluorine. Technical Field The present invention relates to the catalyst technical field; It is the method that a kind of carbonylation of olefin rhodium catalyst waste reaction solution directly prepares using rhodium complex catalysts.
Properties
- Melting point: 120-125 °C (dec.)(lit.)
- Storage temp.: 2-8°C
- Solubility: It is soluble in an organic or non-aqueous solvent.
- Form: crystal
- Color: red
- Water Solubility: soluble in most organic solvents; insoluble aliphatic hydrocarbons
- Sensitive: Air & Moisture Sensitive.
Structure
Rhodium (Rh) is a critical component of many catalysts for a variety of chemical transformation processes. The molecule consists of two planar Rh(I) centers linked by two bridging chloride ligands and four CO ligands. X-ray crystallography shows that the two Rh(I) centers are square planar with a dihedral angle of 53° between the two RhCl2 planes. The metals are nonbonding. The background of invention carbonylation of olefin is the important homogeneous catalytic reaction by olefin production aldehyde of a class. Classical carbonylation of olefin process units is to be the high-pressure process of the catalyst with the carbonyl cobalt.