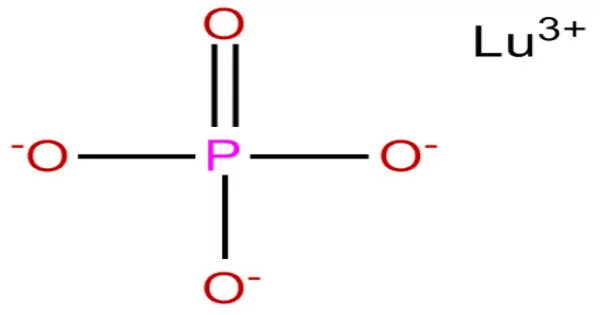
Lutetium phosphide is an inorganic compound of lutetium and phosphorus with the chemical formula LuP. The compound forms dark crystals and does not dissolve in water. Lutetium (Lu) is a rare earth metal and is a part of the lanthanide series on the periodic table. It is known for its high density and is often used in various applications, including research, medical imaging, and the production of certain types of catalysts.
Phosphide compounds typically refer to compounds containing the phosphide ion (P3-). Phosphide compounds can exhibit various properties depending on the specific elements involved and their bonding arrangements.
Synthesis
Heating powdered lutetium and red phosphorus in an inert atmosphere or vacuum:
4Lu + P 4 ⟶ 4 LuP
It can also be formed in the reaction of lutetium and phosphine.
Lutetium is a dense metal, and this property would contribute to the overall density of lutetium phosphide. Lutetium has relatively high melting and boiling points. Lutetium is paramagnetic, meaning it has weak magnetic properties. It is relatively stable in air, but it reacts slowly with water and is soluble in acids.
Physical properties
Lutetium phosphide forms dark cubic crystals, space group Fm3m, cell parameters a = 0.5533 nm, Z = 4. It is stable in air, does not dissolve in water, and reacts actively with nitric acid.
- Chemical formula: LuP
- Molar mass: 205.94
- Appearance: Dark crystals
- Density: 8,1
- Solubility in water: Insoluble
- Crystal structure: cubic
Some phosphides exhibit semiconductor or metallic properties, depending on the specific compound and its structure. Certain phosphide compounds may have interesting optical properties. Phosphides can react with acids to produce phosphine gas.
Uses
The compound is a semiconductor used in high-power, high-frequency applications, and in laser diodes. It is also used in gamma radiation detectors due to its ability to absorb radiation.