Transporting hydrogen is an important part of using it as an energy carrier, and developing cost-effective and efficient ways to do it is critical. One of the most efficient methods of transporting hydrogen is via liquid hydrogen (LH2). It is kept at extremely low temperatures (-253°C or -423°F) and pressures. LH2 has a high storage density, making it excellent for long-distance transit. The energy-intensive liquefaction process, on the other hand, can be a disadvantage.
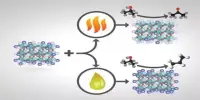
Researchers created a new hydrogen energy carrier material that can store hydrogen energy effectively and potentially more inexpensively. At normal temperatures, each molecule can store one electron from hydrogen for up to three months and can operate as its own catalyst to extract said electron. Furthermore, because the chemical is mostly composed of nickel, its cost is quite modest.
Researchers in Japan have created a novel material capable of storing hydrogen energy in a more efficient and cost-effective manner, as part of the ongoing drive to transition humanity away from fossil fuels and toward more ecologically friendly energy sources. The new hydrogen energy carrier can even store energy at ambient temperature for up to three months. Furthermore, because the material is nickel-based, it is quite inexpensive. The findings were published in Chemistry – A European Journal.
Transporting it in its gaseous state necessitates a significant amount of energy. Another method of storing and conveying it would be to ‘split-up’ the hydrogen atoms into their constituents, electrons, and protons.
Professor Seiji Ogo
As humanity fights the growing climate problem, one option that academics are focusing on is the shift to alternate energy sources such as hydrogen. Kyushu University has been researching techniques to more efficiently use and store hydrogen energy in order to achieve a carbon-neutral society for several decades.
“We have been working on developing new materials that can store and transport hydrogen energy,” explains Professor Seiji Ogo, who led the research team at Kyushu University’s International Institute for Carbon-Neutral Energy Research. “Transporting it in its gaseous state necessitates a significant amount of energy.” Another method of storing and conveying it would be to ‘split-up’ the hydrogen atoms into their constituents, electrons and protons.”
Many candidates have been considered as possible hydrogen energy carriers such as ammonia, formic acid, and metal hydrides. However, the final energy carrier had not yet been established.

“So, we looked to nature for hints. There are a series of enzymes called hydrogenases that catalyze hydrogen into protons and electrons and can store that energy for later use, even at room temperature,” continues Ogo. “By studying these enzymes our team was able to develop a new compound that does exactly that.”
Not only could this novel substance extract and store electrons at normal temperature, but later research revealed that it can also operate as its own catalyst to extract said electron, which was not conceivable with earlier hydrogen energy carriers. The researchers also demonstrated that the energy could be kept for up to three months.
Ogo also emphasizes the compound’s utilization of a low-cost element: nickel. Previously, similar catalysts required expensive metals such as platinum, rhodium, or iridium. Because nickel is now a feasible choice for hydrogen energy storage, it has the potential to lower the cost of future molecules.
The team plans to work with the industrial sector to translate their new discoveries into more practical uses.
“We would also like to work on improving storage time and efficiency as well as investigate the viability of cheaper metals for such compounds,” Ogo goes on to say. “Hopefully our findings will contribute to the goal of decarbonization so that we can build a greener and environmentally friendly future.”