There are several novel synthesis processes that have been developed for the sustainable use of small molecules. One example is the use of renewable feedstocks, such as plant-based materials, instead of fossil fuels as the starting materials for chemical synthesis. Another example is the use of catalysts and enzymes to reduce the amount of energy required for chemical reactions and to increase the selectivity of the reactions. Additionally, the use of continuous flow processes and microreactors can also increase the efficiency and sustainability of small molecule synthesis.
Another example is the use of green chemistry principles, such as the use of non-toxic solvents and reagents, the reduction of waste, the use of atom economy, and the efficient use of resources in chemical reactions.
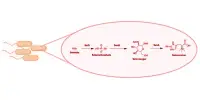
The reaction principle does not necessitate the use of expensive or toxic metals. Furthermore, it allows for the production and subsequent transformation of a chemical reagent that was previously only known as an unstable, transient species. Researchers have discovered a new synthetic pathway for producing anionic ketenes, a specific organic compound, from the simple molecule carbon monoxide (CO).
Researchers at Ruhr University Bochum, Germany, have discovered a new synthetic pathway for producing anionic ketenes from the simple molecule carbon monoxide (CO). Previously, these were only known as reactive intermediates and thus could not be used as defined reagents. The Bochum-based scientists created anionic ketenes that were stable enough to isolate. Unlike previous methods, which can produce higher-value compounds from simple molecules, this approach doesn’t require any expensive or toxic metals.
Mike Jörges, Felix Krischer, and Professor Viktoria Däschlein-Gessner from the Ruhr Explore Solvation (RESOLV) Cluster of Excellence published their findings in the journal Science.
Small molecules such as hydrogen, carbon dioxide or carbon monoxide often occur as by-products of large-scale technical processes or are easily accessible from renewable raw materials.
Däschlein-Gessner
“Small molecules such as hydrogen, carbon dioxide or carbon monoxide often occur as by-products of large-scale technical processes or are easily accessible from renewable raw materials,” explains Däschlein-Gessner. “Since they are readily available, they are of interest as synthesis building blocks to obtain essential feedstock or fine chemicals such as agrochemicals or pharmaceuticals. It’s a promising avenue for the development of sustainable synthesis processes.”
It is worth noting that the use of computational techniques such as computational chemistry and machine learning can also help to design more efficient and sustainable synthetic routes for small molecules.

Conversion without transition metals
To activate small molecules and convert them into more complex compounds, certain metals known as transition metals due to their position in the periodic table subgroups are usually required. These are frequently precious metals, which are scarce and sometimes toxic. Only a few compounds derived from the abundant main group elements have so far been able to activate small molecules. This is also true for carbon monoxide. Furthermore, reactions using CO as a building block are less selective: in addition to the desired high-value compounds, undesirable by-products are frequently formed.
The researchers from the Chair of Inorganic Chemistry II at Ruhr University Bochum have now used simple phosphorus compounds, i.e. so-called ylides, in combination with sodium or potassium bases to tackle this challenge. By realizing a previously unknown transition metal-like reaction mode of these carbon compounds, they enabled the efficient incorporation of CO into larger molecules with a high degree of selectivity.
Just like a molecular model kit
“These transformations’ selectivities are impressive, especially when compared to other synthesis methods,” says Viktoria Däschlein-Gessner. “This is due to the anions’ stability, which is caused by their unique electronic structure. They can be specifically converted with other molecules, just like in a molecular model kit, allowing for the rapid construction of various, complex structures.”
The Bochum group plans to investigate the reaction principle and the potential of anionic ketenes in future research directions. “The reaction mode of the phosphorus compounds is groundbreaking for developing additional processes to use sustainable building blocks like CO,” Däschlein-Gessner emphasizes. “We are also confident that anionic ketenes have even greater potential for synthetic chemistry than we have demonstrated thus far.”