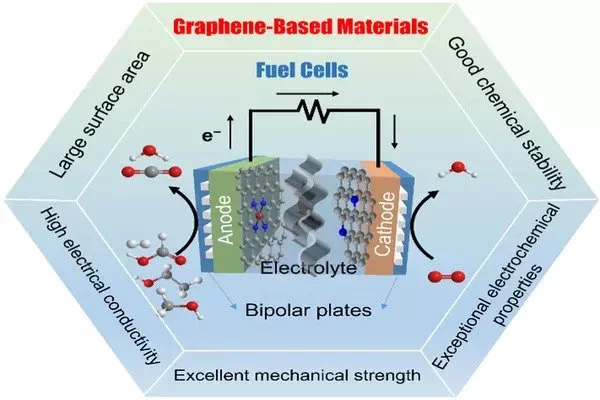
As people seek cleaner transportation, hydrogen fuel cells for automotive applications are becoming more popular. However, the technology has historically been hampered by component cost and longevity issues, which have prevented it from gaining a firm foothold in the automotive industry.
A research team led by Prof. Gao Minrui and Prof. Yang Qing from the University of Science and Technology of China (USTC) developed a new catalyst with excellent CO-tolerance and a low cost in a study published in Angewandte Chemie International Edition, resulting in improved fuel cell performance.
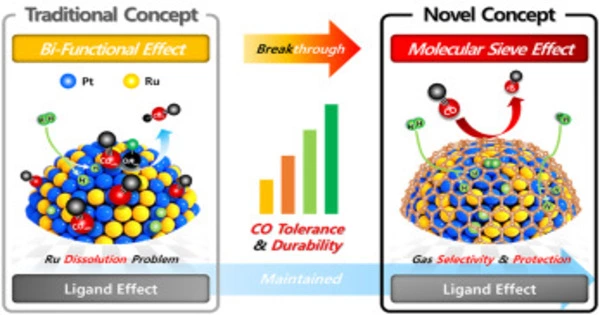
Hydrogen-oxygen fuel cells, with their high specific energy and zero emissions, play an important role in China’s carbon peaking and carbon neutrality goals. However, the commercially used platinum on carbon (Pt/C) catalyst in full cells is susceptible to carbon monoxide (CO) poisoning. Poisoning, in particular, when combined with the slow rate of hydrogen oxidation reaction (HOR) in anion-exchange membrane fuel cells (AEMFCs), significantly reduces the cells’ performance.
The research has not only enabled the reduction of CO poisoning in AEMFCs, but it has also shed light on the development of other non-noble metal catalysts for more efficient fuel-cell applications.
The researchers discovered that when cobalt was added to a molybdenum-nickel alloy, the CO adsorption energy at the nickel site was significantly reduced (MoNi4).
To address the issues, researchers incorporated cobalt (Co) into molybdenum-nickel alloy (MoNi4) to create the Co-MoNi4 catalyst, which demonstrated not only excellent HOR activity in alkali, but also high CO tolerance due to the incorporation of Co bringing electron deficient nickel sites, resulting in less dCO 2* back-donation and thus weakened CO binding.
In the rotating disk electrode test, the Co-MoNi4’s activity only decayed a little after 10,000 cycles in the presence of 500 parts per million (ppm) CO. The further test of the performance of AEMFCs, equipped with the catalyst, revealed a peak power density of 394 mW cm-2 in the presence of 250 ppm CO, exceeding the 209 mW cm-2 of the Pt/C catalyst, while in pure H2 the number reached 525 mW cm-2.
The research has not only enabled the reduction of CO poisoning in AEMFCs, but it has also shed light on the development of other non-noble metal catalysts for more efficient fuel-cell applications.
PEMFCs (hydrogen-oxygen polymer electrolyte membrane fuel cells) electrochemically react hydrogen and oxygen to produce electricity while emitting only water as a byproduct, making them an appealing alternative to gasoline or electric vehicles. A platinum catalyst is typically used in fuel cells to accelerate the reaction at the oxygen electrode, but platinum is expensive. The start-up and operation of the fuel cell also introduces side reactions that reduce the catalyst’s efficacy.
The researchers demonstrated a high-performance platinum-group metal (PGM)-free catalyst that effectively addresses the twin issues of cost and durability. They created a catalytically active cobalt-nitrogen-carbon catalyst with an ultra-fine dispersion of catalytically active sites. They demonstrated that the catalyst was four times more stable than the best PGM-free iron-nitrogen-carbon catalysts and approached the performance of state-of-the-art PGM-free catalysts.