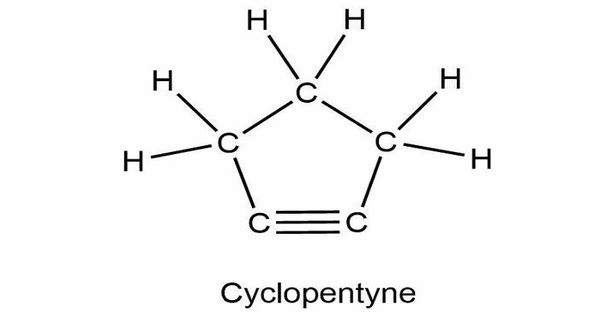
Cyclopentyne, a cyclic hydrocarbon, with the chemical formula C₅H₆. It is a cycloalkyne with five carbon atoms in its ring. It is a highly reactive molecule because it contains a triple bond between two carbon atoms in the five-membered ring. This chemical has a highly strained structure due to the ideal bond angle of 180° at each alkyne atom and the structural necessity that the bonds form a ring. The triple bond is also very reactive. The triple bond is easily converted into [2+2] and [4+2] cycloaddition processes.
Cyclopentyne is an example of a cycloalkyne, which is distinguished by its unique reactivity when compared to alkene and alkyne counterparts. Because of its high reactivity, cyclopentyne is commonly utilized as a reactant in chemical synthesis to produce a variety of organic compounds. However, its volatility makes it difficult to handle and store, necessitating specialised techniques and circumstances.
Unlike benzyne, which undergoes a [2+2] addition with loss of stereochemistry at the alkene partner, cyclopentyne reacts with alkenes while maintaining the geometry of the partner, demonstrating the importance of orbital symmetry even for highly reactive compounds.
The structure can create a π complex with lithium cations, which influences cycloaddition reactivity. It can even interact strongly with copper species to generate a new type of metallacycle.
Cyclopentyne is particularly unstable because to the strain caused by the triple bond in a tiny ring, and it rapidly undergoes numerous chemical processes such as polymerization and combustion. It is largely of interest in organic chemistry because of its unusual reactivity and potential synthetic uses, albeit its practical use is limited due to its instability.