A membrane separation system is a process that separates two or more substances using a selectively permeable membrane. The membrane allows certain substances to pass through while blocking others based on their size, shape, and chemical properties.
Hafnium (Hf) has no independent ore in nature; it is always closely symbiotic with zirconium (Zr) in a homogeneous form, and accounts for only approximately 2% of Zr.
Zr and Hf have similar physical and chemical properties. Their nuclear properties such as neutron absorption cross section are completely opposite.
Additionally, the outer electronic structures of Zr and Hf are comparable, and the atomic and ionic radii are nearly identical due to the contraction of the lanthanide series. As a result, it is highly challenging to separate Zr and Hf, particularly while Hf is being enriched.
The research team at the Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences, under the direction of Prof. Yang Fan, developed an advanced ion-imprinted membrane (IIMs) separation system to achieve highly effective separation and enrichment of Hf in a study that was published in the Journal of Membrane Science.
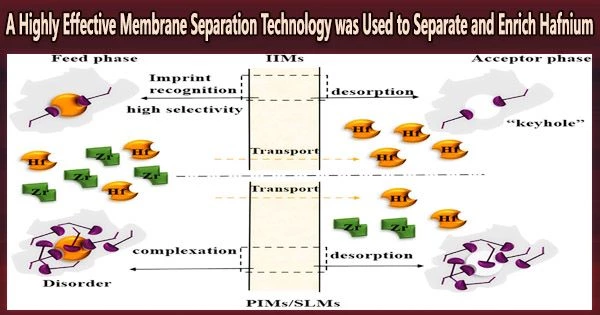
The researchers prepared ion-imprinted membranes (IIMs) using Hf ions as the imprinted ion, N, N-di-2-ethylhexyl diglycolamic acid (D2EHDGAA) as the carrier molecule, and cellulose triacetate (CTA) as the base polymer, and IIMs were applied to the separation and enrichment of Hf from zirconium oxychloride solution.
They found that IIMs can increase CHf/CZr from 2.33:100 to 33.33:100 within 1 h compared with polymer inclusion membranes (PIMs) and ionic liquid supported liquid membranes (SLMs), which increased by 3.33 and 3.67 times, respectively.
The separation factor (SF) was increased by 1.13 and 1.40 times, respectively. The separation-regeneration cycle experiments revealed that the stability of IIMs was reasonably good and that the recovery rate of Hf was 29.2%.
The researchers also discovered that IIMs, PIMs, and SLMs can extract and selectively separate Hf ions from zirconium oxychloride solution, and that the selectivity to Hf declines from IIMs > PIMs > SLMs in that order. This selectivity is primarily owing to the complimentary functional groups and mutual structural matching between the imprinted holes of IIMs and Hf template ions.
According to this study, IIMs may offer a lot of potential for industrial use as a novel concept for the effective separation and enrichment of Hf from zirconium oxychloride solution.