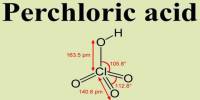
Cobalt is a chemical element with the symbol Co and atomic number 27. It is a ferromagnetic metal of Group 9 (VIIIb) of the periodic table, used especially for heat-resistant and magnetic alloys. Like nickel, cobalt is found in the Earth’s crust only in chemically combined form, save for small deposits found in alloys of natural meteoric iron. It is a hard ferromagnetic, silver-white, hard, lustrous, brittle element. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal. The element is active chemically, forming many compounds.
The word cobalt is derived from the German ‘kobold’, meaning goblin or elf. The image of the cobalt below is by Ben Mills.
Properties
- atomic number: 27
- atomic weight: 58.9332
- melting point: 1,495 °C (2,723 °F)
- boiling point: 2,870 °C (5,198 °F)
- density: 8.9 gram/cm3 at 20 °C (68 °F)
- oxidation states: +2, +3
Cobalt, though widely dispersed, makes up only 0.001 percent of Earth’s crust. It is stable in air and unaffected by water but is slowly attacked by dilute acids. Cobalt-based blue pigments (cobalt blue) have been used since ancient times for jewelry and paints, and to impart a distinctive blue tint to glass, but the color was later thought to be due to the known metal bismuth. Cobalt blue is an important part of artists’ palette and is used bu craft workers in porcelain, pottery, stained glass, tiles, and enamel jewelry. Miners had long used the name kobold ore for some of the blue-pigment-producing minerals; they were so named because they were poor in known metals, and gave poisonous arsenic-containing fumes when smelted. In 1735, such ores were found to be reducible to a new metal (the first discovered since ancient times), and this was ultimately named for the kobold.
Cobalt is primarily used in lithium-ion batteries, and in the manufacture of magnetic, wear-resistant and high-strength alloys. It is an element that occurs naturally in the environment in air, water, soil, rocks, plants, and animals. The compounds cobalt silicate and cobalt(II) aluminate (CoAl2O4, cobalt blue) give a distinctive deep blue color to glass, ceramics, inks, paints, and varnishes. In its compounds, cobalt nearly always exhibits a +2 or +3 oxidation state, although states of +4, +1, 0, and −1 are known. The magnetic properties of cobalt are even more obvious in alloys. The melting point of cobalt metal is 1,493°C (2,719°F), and the boiling point is about 3,100°C (5,600°F). The density is 8.9 grams per cubic centimeter.
Cobalt occurs naturally as only one stable isotope, cobalt-59. Cobalt-60 is a commercially important radioisotope, used as a radioactive tracer and for the production of high-energy gamma rays. The radioactive isotopes, cobalt-60, is used in medical treatment and also to irradiate food, in order to preserve the food and protect the consumer. Its metal is sometimes used in electroplating because of its attractive appearance, hardness, and resistance to corrosion. Its salts have been used for centuries to produce brilliant blue colors in paint, porcelain, glass, pottery, and enamels.