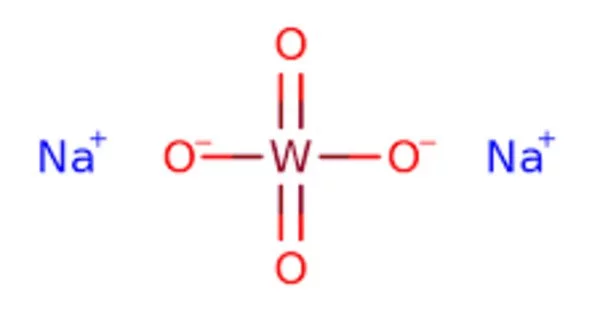
Sodium tungstate is the inorganic compound with the formula Na2WO4. This white, water-soluble solid is the sodium salt of tungstic acid. It is useful as a source of tungsten for chemical synthesis. It is an intermediate in the conversion of tungsten ores to the metal. One of the most significant uses of sodium tungstate is as a precursor to tungsten metal. It is used in the production of tungsten metal and its alloys because of its ability to dissolve easily in molten metals.
Properties
It is a white, water-soluble solid that is commonly used in analytical chemistry as a reagent for the detection of various metal ions. It is an important compound with a variety of uses in different fields.
- Chemical formula: Na2WO4
- Molar mass: 293.82 g/mol
- Appearance: White rhombohedral crystals
- Density: 4.179 g/cm3 (anhydrous); 3.25 g/cm3 (dihydrate)
- Melting point: 698 °C (1,288 °F; 971 K)
- Solubility in water: 57.5 g/100 mL (0 °C); 96.9 g/100 mL (100 °C)
- Solubility: slightly soluble in ammonia; insoluble in alcohol, acid
- Crystal structure: Rhombic (anhydrous); orthorhombic (dihydrate)

Preparation and structure
Sodium tungstate can be prepared by reacting tungsten oxide with sodium hydroxide in water. It is also a byproduct of the production of tungsten metal. It is obtained by digestion of tungsten ores, the economically important representatives of which are tungstates, in base. Illustrative is the extraction of sodium tungstate from wolframite:
Fe/MnWO4 + 2 NaOH + 2 H2O → Na2WO4·2H2O + Fe/Mn(OH)2
Scheelite is treated similarly using sodium carbonate.
Sodium tungstate can also be produced by treating tungsten carbide with a mixture of sodium nitrate and sodium hydroxide in a fusion process which overcomes the high exothermicity of the reaction involved.
Several polymorphs of sodium tungstate are known, three at only one atmosphere pressure. They feature tetrahedral orthotungstate dianions but differ in the packing motif. The WO2−4 anion adopts a structure like sulfate (SO2−4).
Reactions
Treatment of sodium tungstate with hydrochloric acid gives the tungsten trioxide or its acidic hydrates:
Na2WO4 + 2 HCl → WO3 + 2 NaCl + H2O
Na2WO4 + 2 HCl → WO3·H2O + 2 NaCl
This reaction can be reversed using aqueous sodium hydroxide
Uses
Sodium tungstate is primarily used as an intermediate in the extraction of tungsten from its ores, almost all of which are tungstates. Otherwise, sodium tungstate has only a few uses. Sodium tungstate is used as a catalyst in organic chemistry for the epoxidation of alkenes and the oxidation of alcohols to aldehydes or ketones. It has anti-diabetic properties.
In density separation, sodium and lithium metatungstate solutions are used. These solutions are less toxic than bromoform and methylene iodide, but they still have densities in the range of naturally coupled minerals.
In addition to its use in analytical chemistry, sodium tungstate has a number of other applications. It is used as a corrosion inhibitor for steel, as a flame retardant for textiles, and as a catalyst in various chemical reactions. It is also used in the production of tungsten powder and other tungsten compounds, and it is sometimes used as a substitute for lead in certain applications due to its high density.
Safety
However, it is important to note that sodium tungstate can be toxic if ingested or inhaled, and it may cause skin and eye irritation upon contact. It should be handled with care and disposed of properly according to local regulations.