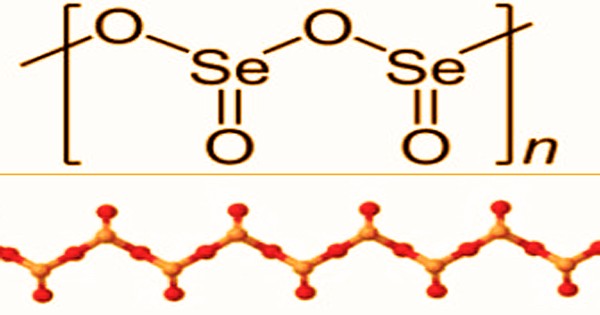
Selenium dioxide is a pungent sour-smelling white or creamy-white volatile lustrous crystal or crystalline powder. The chemical compound with the formula SeO2 is what it is. One of the most commonly found selenium compounds is this colorless solid. When selenium is burned in oxygen, it forms a long, straight polymer with a chain that alternates between selenium and oxygen atoms, unlike other oxides. This means that each selenium atom, with the exception of those at the chain’s ends, is bound to three oxygen atoms, one with a double bond that runs perpendicular to the chain and a pair on either side with single bonds.
When selenium dioxide is heated with carbon and other reducing agents, it is reduced to selenium metal. Selenium dioxide forms selenium, nitrogen, and water when heated with ammonia:
3SeO2 + NH3 → 3Se + 2N2 + 6H2O
Solid SeO2 is a one-dimensional polymer with alternating selenium and oxygen atoms in its chain. Each Se atom is pyramidal and has an oxide group at the end. Selenium dioxide is a powder or crystals that ranges from yellowish white to reddish in color. It is hygroscopic and soluble in water, absorbing moisture or water from the air. Heavy oxidizing agents, reducing agents, strong acids, ammonia, organics, and phosphorus trichloride are incompatible with selenium oxide.
When heated to around 350°C, selenium dioxide transforms into a greenish yellow vapour with a distinct odor that has been characterized as vile and smelling like rotting horseradish. It forms selenous acid when dissolved in water, which is how the oxide is commonly used in reactions. By heating selenium with oxygen and nitrogen dioxide, selenium dioxide can be produced. In the presence of excess oxygen, nitrogen dioxide would be converted to pentoxide, but selenium dioxide would be converted to trioxide:
2Se + 3O2 + 4NO2 → 2SeO2 + 2N2O5
The oxidation of selenium by nitric acid can also produce selenium dioxide. The terminal Se-O gap is 162 pm, and the bridging Se-O bond lengths are 179 pm. Along the polymer chain, the relative stereochemistry at Se alternates (syndiotactic). In the context of glassmaking, selenium dioxide is a striking colorant. The material is white to yellowish pink in color, but it gives glass a rich, red tint. When applied to the melt in small amounts, it can counteract the blue tint caused by traces of cobalt in the silica used to make the glass, and on a larger scale, it can give the glass a glorious deep red color.
Selenium reacts with oxygen to form a variety of white or colorless crystals, as well as certain selenium compounds that are gases. Ingestion of the compound is poisonous. The symptoms of selenium dioxide poisoning are close to those of selenium metal poisoning. The vapors of selenium dioxide are extremely irritating to the skin, nose, and respiratory tract. Selenium dioxide exists as dimers and other oligomeric species in the gas phase, although it is monomeric at higher temperatures. With a bond length of 161 pm, the monomeric shape has a bent structure similar to sulfur dioxide.
Selenium dioxide (SeO2) is made by burning selenium in an air or oxygen stream and, optionally, passing it through a catalyst or oxidizing it with nitric acid to selenous acid, then heating it to dryness. The trimer (Se(O)O)3 is formed when SeO2 is dissolved in selenium oxydichloride. a single molecule The dipole moment of 2.62 D points from the midpoint of the two oxygen atoms to the selenium atom, making SeO2 a polar molecule.
If selenium dioxide, SeO2, can be made by heating selenious acid, H2SeO3, the compound can also be made by heating the element with oxygen enabled by passage through HNO3. Selenium dioxide is easily reduced to elemental selenium by SO2 at 315–317 °C (599–603 °F). When a selenium dioxide solution is combined with concentrated sulfuric acid, it releases Mecke reagent, which is used by a number of organizations to provide spot checks for a variety of illicit drugs, including LSD and morphine (as well as more common substances including codeine and sugar), since it undergoes a color shift that suggests the presence of the drug.
Selenium dioxide is made by burning selenium in air or reacting it with nitric acid or hydrogen peroxide, but the dehydration of selenous acid is perhaps the most convenient method. Added to etching baths, decorative paint forming on galvanized iron and nonferrous metals and alloys, and corrosion-protective coatings in magnesium alloys; drug synthesis; used in the manufacture of cortisone and nicotinic acid (niacin).
Downeyite, a natural source of selenium dioxide, is a very rare mineral. It’s just present in a few burning coal dumps. Selenium dioxide (SeO2) is used in lubricating oils and grease as an oxidizing agent, a catalyst, and an antioxidant. Selenium dioxide has been used in the manufacture of selenide semiconductor nanocrystals, which have exciting potential applications in the formation of quantum dots: semiconductors small enough to undergo quantum effects and being studied for use in quantum computers, specialist transistors, and tiny lasers and LEDs.
Selenium dioxide is used to make selenium compounds, as an oxidizing agent, in paint and ink pigments, in metal “blueing” and etching, as a chemical catalyst, in photographic toners, in electric and photoelectric components, and more. The starting material for selenium is reduced to selenium, which precipitates as a red amorphous solid that can be quickly filtered off. The appearance of selenium in the urine and a garlic-like odor on the breath are also signs of selenium absorption. Dust inhalation can cause bronchial spasms, asphyxiation symptoms, and pneumonitis. Sternal discomfort, cough, nausea, pallor, coated tongue, stomach disorders, nervousness, and conjunctivitis are some of the acute symptoms of ingestion. Contact with the eyes irritates them.
Inorganic oxidizer selenium dioxide reacts violently with reducing agents including hydrides, nitrides, alkali metals, and sulfides. When exposed to strong acids, toxic hydrogen selenide gas can be released. It’s used in small amounts to mask the color caused by iron impurities, resulting in (apparently) colorless glass. It produces a deep ruby red color when used in greater amounts. Some cold-bluing solutions contain selenium dioxide as an active ingredient.
Information Sources: