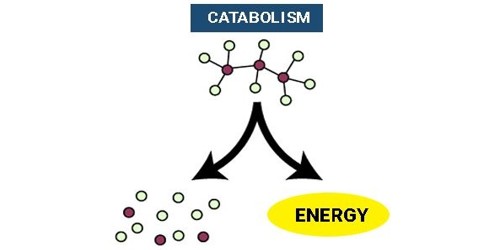
Catabolism is a kind of metabolism and is a process in which molecules turn into smaller units and release energy. It is the part of the metabolism responsible for breaking complex molecules down into smaller molecules. Enzymes take big molecules and break them apart to make smaller molecules. In most cases, the released energies are used to form ATP. Catabolism, therefore, provides the chemical energy necessary for the maintenance and growth of cells. It is the breakdown of complex molecules into simpler ones. These reactions release energy. It expresses the relative part of the activity of catabolism of the organism in relation to its anabolic activity. It is exergonic, meaning it releases heat and works via hydrolysis and oxidation.
During catabolism, energy is released from the bonds of the large molecules being broken down. It is the breaking-down aspect of metabolism, whereas anabolism is the building-up aspect. Part of the chemical energy released during catabolic processes is conserved in the form of energy-rich compounds (e.g., adenosine triphosphate [ATP]). An example of catabolism is glycolysis. This process is almost the reverse of gluconeogenesis. Catabolic processes are thermodynamically favorable and spontaneous, so cells use them to generate energy or to fuel anabolism. For example:
- During cellular respiration, glucose and oxygen react to yield carbon dioxide and water: C6H12O6 + 6O2 → 6CO2 + 6H2O
- In cells, hydroxide peroxide decomposes into water and oxygen: 2H2O2 → 2H2O + O2