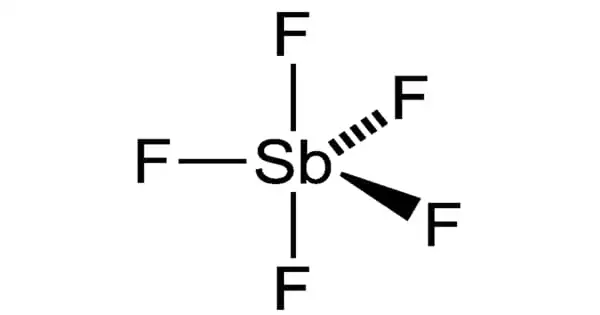
The inorganic chemical with the formula SbF5 is antimony pentafluoride. This colorless, viscous liquid is a useful Lewis acid and a component of the superacid fluoroantimonic acid, which is created by mixing liquid HF in a 2:1 ratio with liquid SbF5. Its Lewis acidity and propensity to react with practically all known chemicals make it unique. It is employed in the production of other chemicals as well as as a catalyst in the production of other chemicals.
It has the appearance of a colorless, greasy liquid. Fumes cause irritation to the eyes and mucous membranes. It is poisonous. It corrodes metals and flesh. It is exceedingly harmful to tissue, and its burns can lead to gangrene.
Properties
- Chemical formula: SbF5
- Molar mass: 216.74 g/mol
- Appearance: colorless oily liquid hygroscopic
- Odor: pungent
- Density: 2.99 g/cm3
- Melting point: 8.3 °C (46.9 °F; 281.4 K)
- Boiling point: 149.5 °C (301.1 °F; 422.6 K)
- Solubility in water: Reacts
- Solubility: soluble in KF, liquid SO2
Preparation
The reaction of antimony pentachloride with anhydrous hydrogen fluoride yields antimony pentafluoride. Antimony trifluoride and fluorine can also be used to make it. Antimony pentafluoride was also utilized in the first chemical process found to create fluorine gas from fluoride compounds. Antimony Pentafluoride is a water-insoluble Antimony source used in oxygen-sensitive applications like metal manufacturing. Most quantities of antimony pentafluoride are readily obtainable.
Antimony pentafluoride is prepared by the reaction of antimony pentachloride with anhydrous hydrogen fluoride:
SbCl5 + 5 HF → SbF5 + 5 HCl
It can also be prepared from antimony trifluoride and fluorine.
Structure and
In the gas phase, SbF5 adopts a trigonal bipyramidal structure of D3h point group symmetry (see picture). The material adopts a more complicated structure in the liquid and solid states. The liquid contains polymers wherein each Sb is octahedral, the structure being described with the formula [SbF4(μ-F)2]n ((μ-F) denotes the fact that fluoride centres bridge two Sb centres). The crystalline material is a tetramer, meaning that it has the formula [SbF4(μ-F)]4. The Sb-F bonds are 2.02 Å within the eight-membered Sb4F4 ring; the remaining fluoride ligands radiating from the four Sb centers are shorter at 1.82 Å.
Chemical reactions
In the solid and liquid states, the related species PF5 and AsF5 are monomeric, most likely due to the lower sizes of the core atoms, which limits their coordination number. BiF5 is a kind of polymer. SbF5 increases the oxidizing power of F2 in the same way as it increases the Brnsted acidity of HF. The oxidation of oxygen exemplifies this effect:
2 SbF5 + F2 + 2 O2 → 2 [O2]+[SbF6]–
Antimony pentafluoride has also been used in the first discovered chemical reaction that produces fluorine gas from fluoride compounds:
4 SbF5 + 2 K2MnF6 → 4 KSbF6 + 2 MnF3 + F2
The driving force for this reaction is the high affinity of SbF5 for F−, which is the same property that recommends the use of SbF5 to generate superacids.
Safety
SbF5 reacts violently with many compounds, often releasing dangerous hydrogen fluoride. It is corrosive to the skin and eyes.