Other compounds are emerging as key sources of stratospheric ozone depletion as the Earth’s ozone layer recovers from earlier emissions of now-banned CFCs and halons. Scientists in the atmosphere have been looking for the sources of around one-third of the greatest concerns, methyl bromide, and methyl chloride. According to new research, common copper-based compounds produce these chemicals when they interact with soil and ocean, with sunlight increasing production by a factor of ten.
According to a new study from the University of California, Berkeley, copper released into the environment via fungicides, brake pads, antifouling coatings on boats, and other sources may be significantly contributing to stratospheric ozone depletion.
UC Berkeley geochemists show in a report published this week in the journal Nature Communications that copper in soil and saltwater functions as a catalyst to convert organic matter into methyl bromide and methyl chloride, two strong halocarbon chemicals that deplete ozone. The issue is exacerbated by sunlight, which multiplies the creation of these methyl halides by a factor of ten.
The findings help to explain, at least in part, the origin of much of the methyl bromide and methyl chloride in the stratosphere. Since the 1989 global ban on CFC refrigerants and brominated halons used in fire extinguishers, these methyl halides have become the new primary producers of ozone-depleting bromine and chlorine in the stratosphere.
“How can we ensure that methyl bromide and methyl chloride are lowered alongside CFCs if we don’t know where they come from?” Robert Rhew, a UC Berkeley professor of geography as well as environmental science, policy, and management, is the paper’s senior author. “By 2050, we should be back to somewhat normal ozone levels, but things like ongoing methyl bromide and methyl chloride emissions are stumbling blocks on the road to recovery. Copper usage in the environment is expected to rise quickly in the coming years, and this should be taken into account when forecasting future halogen load and ozone recovery.”
We were shocked that when we added copper sulfate to soil, it produced a large number of methyl halides. The experiment was subsequently repeated using seawater, which produced an astonishing amount of methyl halides as well.
Professor Robert Rhew
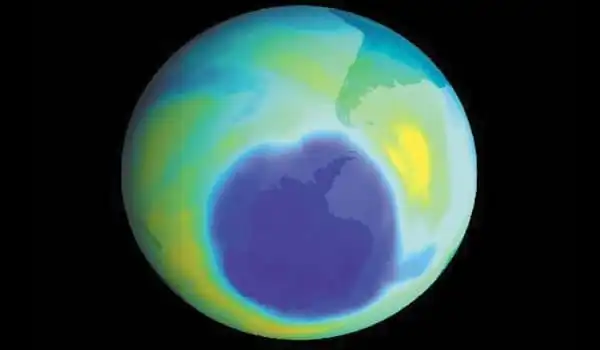
The ozone layer protects us from cancer-causing ultraviolet light from the sun, but compounds containing chlorine and bromine, such as CFCs and halons, were discovered in the 1980s to erode the ozone layer, generating thinner stratospheric layers that let in more of the deadly radiation. Despite a ban on the production of CFCs and halons, the primary producers of halogens, the ozone layer has yet to recover. According to Rhew, the ozone hole over Antarctica was about as terrible as it’s ever been last year.
The ozone hole’s endurance is mostly owing to the persistence of banned ozone-depleting substances, which take decades to disperse in the stratosphere. However, some ozone-depleting substances continue to be emitted. Among the major contributors, today are methyl chloride and methyl bromide. One atom of bromine is 50 times more destructive to ozone than one atom of chlorine.
Despite the fact that methyl bromide is no longer utilized as an agricultural soil fumigant, it is still employed as a pesticide for agricultural product quarantine and pre-shipment. And methyl chloride is used as a chemical feedstock, despite the fact that the majority of its emissions are thought to be from biomass burning or natural sources. However, the overall amount of these methyl halides created each year still does not tally up to the observed yearly addition of these compounds to the atmosphere, which has perplexed scientists for more than 20 years.
According to Rhew, about one-third of the methyl bromide and methyl chloride in the atmosphere comes from unknown sources. The new findings suggest that copper is an important, if not the major, source of the missing methyl bromide and methyl chloride.
“We’ve prohibited methyl bromide, but are other environmental changes causing enormous amounts of this substance to be released into the atmosphere? With the increased use of copper, it appears that copper-catalyzed production is also becoming more popular” Rhew stated.
Yi Jiao, the first author and a former UC Berkeley doctoral student who is now a postdoctoral fellow at the University of Copenhagen in Denmark, noted that copper compounds are allowed on organic crops, a legacy of its use in farming since the 1700s, including as a major antifungal agent in the Bourdeaux mixture used in France since the 1880s to prevent downy mildew on grapes. Because of this history, copper pollution of soils is a big issue in Europe today. The ozone-depleting power of copper is another cause for concern, the authors said.
“Please keep in mind that organic agriculture is not a big contributor to ozone depletion. Copper-based fungicides, on the other hand, appear to have atmospheric side effects that should be evaluated in terms of total environmental impact” This week, Jiao tweeted. “Given the ubiquitous usage of copper in the environment, this potentially expanding impact should be taken into account when forecasting future halogen load and ozone recovery.”
Copper + soil + sunlight = methyl halides
A series of research studies done by UC Berkeley undergraduate scholars first established the link between copper and methyl halides. Rhew requested them to investigate the effects of metal ions, beginning with the replication of previously published work on iron in soils. Rhew then requested them to explore a new metal, copper, in the form of copper sulfate, one of the most prevalent copper compounds used today, after this produced modest amounts of methyl halides.
“We reproduced the iron experiment and then thought, ‘Let’s look at a different transition metal, like copper, and see whether it has a similar effect,'” the researchers said. Rhew stated. “We were shocked that when we added copper sulfate to soil, it produced a large number of methyl halides. The experiment was subsequently repeated using seawater, which produced an astonishing amount of methyl halides as well. So we knew there was a novel process at work, but we only had a few pieces of the puzzle until Yi did a series of creative experiments to piece it all together.”
Jiao and Rhew devised more in-depth studies, collecting soil samples from the Oxford Tract, an agricultural research area near the UC Berkeley campus, and subjected them to various treatments, including varying quantities of copper and oxidants. While copper alone produced some methyl bromide and methyl chloride in soil and seawater, the addition of sunlight and/or hydrogen peroxide – which is produced in soil by microbes or sunlight – produced more than five times the amount of methyl halides and extended copper activity from about a week to between two and three weeks.
Yi sterilized the soil, which increased the amount of methyl halide produced even further. However, after removing all organic material, soil incubated with copper created no methyl halides. This prompted him to concentrate on compounds such as catechol and guaiacol, which are frequently used as proxies for soil organic carbon since they both have a phenol ring structure similar to that found in organic matter.
Increasing the concentrations of copper sulfate or hydrogen peroxide in catechol-halide solutions boosted methyl halide emissions, whereas emissions were near zero when any of these substrates was absent. Following that, Yi discovered that sunlight, like hydrogen peroxide, boosted methyl halide synthesis. Exposing copper-adjusted solutions to sunshine enhanced emissions fourfold in seawater.
The researchers assume that Cu(II), a common type of copper ion, is oxidizing organic material to generate methyl radicals, which readily react with chlorine and other halogens in soil or seawater to form methyl halides. Both sunlight and hydrogen peroxide subsequently reoxidize the copper, converting it from cuprous (I) to cupric (II) so that it can operate to make new methyl halides.
“We ran a back-of-the-envelope calculation to see what impact copper sulfate might have and found that it could be responsible for 4.1 gigagrams of methyl bromide per year, which is around 10% of the missing source,” Rhew explained. “That’s a lot of money, and that’s only for copper sulfate. Another copper chemical, copper hydroxide, is possibly more extensively utilized. So far, we have just scratched the surface of our understanding of copper’s impact on halocarbon chemistry.”
Jiao further pointed out that this does not account for the potential oceanic emissions related with copper in runoff. According to Rhew, much more research is needed to understand which copper compounds are the most potent makers of methyl halides in soil and the ocean, as well as how much is actually created.
“There’s a lot of halide in soils, and a lot of organic matter in the soil, so the magic component is copper, which is renewed by sunlight,” he explained. “This has opened up a whole new area of investigation into the role of copper in the environment.”