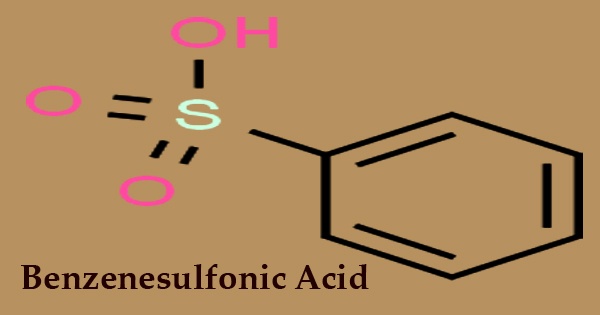
The simplest member of the class of benzene sulfonic acids consisting of benzene carrying a single sulfon group is benzenesulfonic acid (conjugate base benzenesulfonate). With the formula C6H5SO3H, it is an organosulfur compound; it is the simplest aromatic sulfonic acid. It is a benzenesulfonate conjugate acid that forms colorless, delicate sheet crystals or a white waxy solid that is soluble in water and ethanol, partially soluble in benzene, and insoluble in carbon disulfide and diethyl ether. Sometimes in the form of alkali metal salts, it is deposited. Strongly acidic is the aqueous solution.
It is a white crystalline acid which is formed by benzene sulfonation. The 3 position is led to any additional substitution on the benzene ring. Benzenesulfonic acid is prepared using concentrated sulfuric acid from benzene sulfonation:
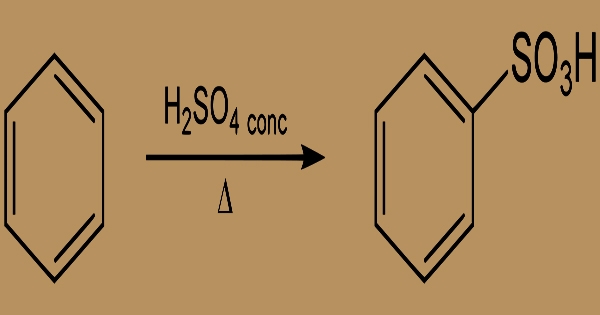
Aromatic sulfonation of benzene
By treating benzene with concentrated sulphuric acid, benzene sulfonic acid is formed. As detergents are used in alkyl derivatives. The reactions typical of other aromatic sulfonic acids, producing sulfonamides, sulfonyl chloride, and esters, are seen in this acid. Above 220°C, the sulfonation is reversed. Benzene sulfonic acid alkali metal salt was once commonly used in phenol processing:
C6H5SO3Na + 2 NaOH → C6H5ONa + Na2SO3
C6H5ONa + HCl → C6H5OH + NaCl
Benzenesulfonic acid anhydride ((C6H5SO2)2O) is supplied through dehydration with phosphorus pentoxide. The phosphorus pentachloride is converted to the corresponding benzenesulfonyl chloride (C6H5SO2Cl). It is a strong acid, dissociated almost completely in water. The key consumption of benzene sulfonic acid is by conversion to other specialty chemicals. As benzene sulfonic acid salts, a number of prescription drugs are prepared and are known as besylates or besilates.
There is no flash point data for this chemical (benzenesulfonic acid), but it is probably combustible. When heated in water above 200°C, Benzenesulfonic acid and related compounds undergo desulfonation. The desulfonation temperature corresponds to the ease of sulfonation:
C6H5SO3H + H2O → C6H6 + H2SO4
In a vacuum desiccator, a 32 percent commercial acid is allowed to crystallize fractionally at room temperature over P2O5, giving essentially colorless delicuescent plates m 52.5o. The crystalline anhydrous acid is deliquescent and should be preserved in the dark over anhydrous Na2SO4 and should be used as it darkens under sunlight in subdued sunlight. As the active ingredient in laundry detergent used in clothes washing machines, benzensulfonic acid is widely used. For conversion to other specialty chemicals, benzenesulfonic acid is also used. By means of a dry chemical, carbon dioxide, or Halon extinguisher, fires involving this compound may be extinguished. Benzenesulfonic acid is also used as a polymer remover stripping agent in a diluted form.
Information Sources: