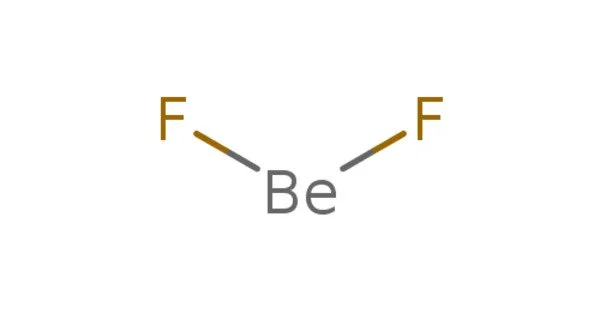
Beryllium fluoride, abbreviated BeF2, is an inorganic chemical. It is a white solid with no odor. This whitish solid is the primary precursor used in the production of beryllium metal. Although its structure is similar to that of quartz, BeF2 is highly soluble in water. It is utilized as a phosphate mimic in biochemistry, notably protein crystallography.
It is an amorphous, hygroscopic solid with a melting point of 800°C. It is water soluble and is utilized in beryllium metallurgy as an alloy with the metal ‘Be’. The structure of solid crystalline BeF2 is silica-like, with beryllium in a four-coordinate position.
Properties
Beryllium fluoride has distinctive optical properties. In the form of fluoroberyllate glass it has the lowest refractive index for a solid at room temperature of 1.275. Its dispersive power is the lowest for a solid at 0.0093, and the nonlinear coefficient is also the lowest at 2 × 10−14.
- Molar mass: 47.01 g/mol hygroscopic
- Appearance: colorless, glassy lumps
- Density: 1.986 g/cm3
- Melting point: 554 °C (1,029 °F; 827 K)
- Boiling point: 1,169 °C (2,136 °F; 1,442 K)
- Solubility in water: very soluble
- Solubility: sparingly soluble in alcohol

Structure and bonding
Solid BeF2 has a structure similar to cristobalite. Be2+ centers are tetrahedral and four-coordinate, whereas fluoride centers are two-coordinate. Be-F bond lengths are around 1.54Å. BeF2 can take on a variety of similar structures to SiO2. BeF2 and AlF3 have an analogue in that they both assume extended structures at low temperatures.
Gas and liquid BeF2
With a Be-F distance of 143 pm, gaseous beryllium fluoride has a linear structure. BeF2 has a vapor pressure of 10 Pa at 686 degrees Celsius, 100 Pa at 767 degrees Celsius, 1 kPa at 869 degrees Celsius, 10 kPa at 999 degrees Celsius, and 100 kPa at 1172 degrees Celsius.
Liquid beryllium fluoride’molecules’ have a changing tetrahedral structure. Furthermore, near the freezing point, the density of liquid BeF2 falls as Be2+ and F ions begin to coordinate more strongly with one another, resulting in the growth of voids between formula units.
Production
The processing of beryllium ores generates impure Be(OH)2. This material reacts with ammonium bifluoride to give ammonium tetrafluoroberyllate:
Be(OH)2 + 2 (NH4)HF2 → (NH4)2BeF4 + 2 H2O
Tetrafluoroberyllate is a robust ion, which allows its purification by precipitation of various impurities as their hydroxides. Heating purified (NH4)2BeF4 gives the desired product:
(NH4)2BeF4 → 2 NH3 + 2 HF + BeF
In general the reactivity of BeF2 ions with fluoride are quite analogous to the reactions of SiO2 with oxides.
Uses
Beryllium fluoride is employed in biochemistry, particularly protein crystallography, since it binds in human tissues in some of the same ways that phosphate does. ADP and beryllium fluoride tend to bind to ATP sites and block protein function, allowing proteins to crystallize in the bound state.
Beryllium fluoride is a fundamental component of the preferred fluoride salt mixture used in liquid-fluoride nuclear reactors. Typically, beryllium fluoride is combined with LiF to generate a base solvent into which uranium and thorium fluorides are added. Beryllium fluoride is chemically extremely stable, and LiF/BeF2 mixes have the lowest melting points and the highest neutronic characteristics of any fluoride salt combination suitable for reactor use.