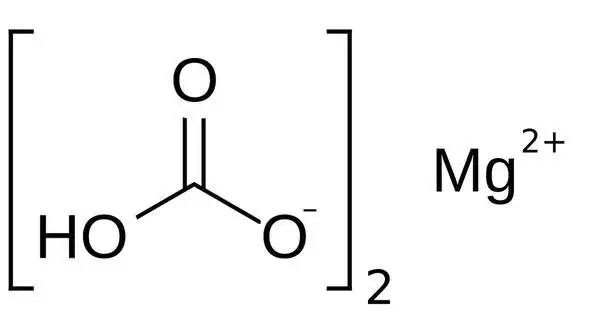
Magnesium bicarbonate, also known as magnesium hydrogencarbonate, Mg(HCO3)2, is the bicarbonate salt of magnesium. In its pure form, it is a rare and unstable chemical. It can be produced by reacting dilute concentrations of carbonic acid (such as seltzer water) and magnesium hydroxide (milk of magnesia). It is formed when magnesium hydroxide (Mg(OH)2) or magnesium oxide (MgO) combines with carbon dioxide (CO2) in water to generate magnesium bicarbonate and water.
It can be prepared through the synthesis of magnesium acetate and sodium bicarbonate:
Mg(CH3COO)2 + 2 NaHCO3 → Mg(HCO3)2 + 2 CH3COONa
Magnesium bicarbonate exists only in aqueous solution. Magnesium does not form solid bicarbonate as does lithium. To produce it, a suspension of magnesium hydroxide is treated with pressurized carbon dioxide, producing a solution of magnesium bicarbonate:
Mg(OH)2 + 2 CO2 → Mg(HCO3)2
Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding magnesium carbonate, carbon dioxide, and water:
Mg2+ + 2 HCO3− → MgCO3 + CO2 + H2O
Solubility
It dissolves in water and becomes more soluble in the presence of carbon dioxide. When dissolved in water, carbon dioxide can react with magnesium carbonate or hydroxide to generate soluble magnesium bicarbonate ions.
Stability
It is a non-stable chemical that decomposes into other compounds in solution. It is more typically found in water as dissolved ions.
Health and Nutrition
Magnesium is a necessary mineral for human health, as it aids in a variety of biological activities such as muscle and nerve function, blood glucose management, and bone health. While magnesium bicarbonate is not a commonly utilized dietary supplement or source of magnesium, magnesium-containing substances such as magnesium citrate or magnesium oxide are.
Water Treatment
It is a component of hard water, which can create scale accumulation in pipes and appliances. Water treatment procedures can be used to soften water by eliminating or reducing the concentration of magnesium and calcium ions.
Chemical Reactions
It can engage in a number of chemical reactions in aqueous solutions. It can, for example, react with acids to produce carbon dioxide gas, water, and magnesium salts. It is sometimes found in natural mineral waters or spring waters, where it is created by the dissolution of magnesium-containing minerals in the presence of carbon dioxide. It is a source of magnesium, which is required for many biological functions in the human body.