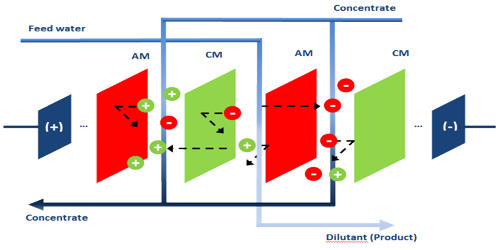
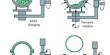
Electrodialysis Reversal (EDR) is an electrochemical charge-driven separation process where dissolved ions are separated through ion permeable membranes under the influence of an electrical potential gradient. EDR is an electrodialysis reversal water desalination membrane process that has been commercially used since the early 1960s. It is a water desalination process in which electricity is applied to electrodes to pull naturally occurring dissolved salts through an ion-exchange membrane to separate the water from the salts. An electric current migrates dissolved salt ions, including fluorides, nitrates, and sulfates, through an electrodialysis stack consisting of alternating layers of cationic and anionic ion-exchange membranes. It involves applying a direct current (DC) electric field to flux positive ions across cation exchange membranes (CEM) in one direction, and negative ions through anion exchange membranes (AEM) in the opposite direction. Periodically (3-4 times per hour), the direction of ion flow is reversed by reversing the polarity of the applied electric current. This reversal acts as a self-cleaning feature that minimizes fouling and prolongs membrane life.
In the reversal process, the polarity of the electrodes is switched at fixed intervals to reduce the formation of scale and subsequent fouling and allow the EDR to achieve higher water recoveries. EDR has advantageous characteristics that constitute it as a success. First is EDR’s ability to perform at very high water recovery sue to its polarity reversal which allows for treatment, without any chemicals, of feeds with concentrated salt scale factors well beyond saturation. EDR is used to treat brackish waters with moderate total dissolved solids (TDS) and waters that have a high scaling potential due to elevated levels of particular contaminants such as barium (Ba) and strontium (Sr). EDR is also effective on high silica (SiO2) feedwaters. The disadvantage of EDR is that it doesn’t remove microorganisms and organic contaminants, thus post-treatment is always necessary if high-quality water is required. EDR has not yet been used to treat these waters but this technology should be suitable. The question is whether EDR would be cost-effective.
EDR is known for its excellence to desalt Ca2+ and dominated brackish groundwater with a higher water recovery rate, an elevated silt density index, the potential for biofouling, hard-to-treat, high hardness, and lower salinity feed waters with the ranges of 200–5000 mg/L TDS. Brackish waters with total dissolved solids (TDS) concentrations less than 10,000 mg/L are extracted from coal-beds in the Wyoming Powder River basin to facilitate the production of coal-bed methane. These waters frequently require treatment before disposal or use.