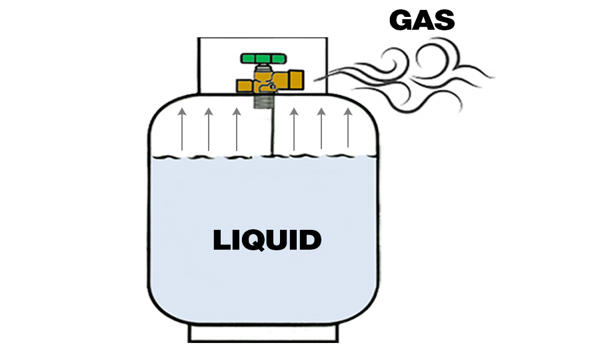
Propane is a three-carbon alkane with the molecular formula C3H8. It is nontoxic, colorless, and virtually odorless; an identifying odor is added so it can be detected. It is a gas at standard temperature and pressure, but compressible to a transportable liquid. It is sometimes known as liquefied petroleum gas, or LPG — is a gas normally compressed and stored as a liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation. It is also used for space and water heating, for cooking, and as fuel for engine applications such as forklifts, farm irrigation engines, fleet vehicles, and buses; however, its applications are rapidly growing due to new technology developments.
Discovered in 1857 by the French chemist Marcellin Berthelot, it became commercially available in the US by 1911. Nearly 97 percent of propane consumed in the United States is produced in North America. Propane is one of a group of liquefied petroleum gases (LP gases). It is produced from both natural gas processing and crude oil refining, in roughly equal amounts from each source. The others include butane, propylene, butadiene, butylene, isobutylene, and mixtures thereof. It is nontoxic, colorless, and virtually odorless. Propane has a lower energy density but burns more cleanly than gasoline and coal. As with natural gas, an identifying odor is added so the gas can be readily detected.
Properties
Propane is a colorless, odorless gas. At normal pressure, it liquefies below its boiling point at −42°C and solidifies below its melting point at −187.7°C. It is primarily a byproduct of domestic natural gas processing, though some propane is produced from crude oil refinement. Propane crystallizes in the space group P21/n. The low space-filling of 58.5 % (at 90 K), due to the bad stacking properties of the molecule, is the reason for the, particularly low melting point. It is a safe and environmentally friendly fuel that is available now and widely used throughout the United States in homes, on farms, on the road, and in industrial and commercial operations.
Density
The density of propane gas at 25 °C (77 °F) is 1.808 kg/m3. The density of liquid propane at 25 °C (77 °F) is 0.493 g/cm3, which is equivalent to 4.11 pounds per U.S. liquid gallon or 493 g/L. Propane expands at 1.5% per 10 °F. Thus, liquid propane has a density of approximately 4.2 pounds per gallon (504 g/L) at 60 °F (15.6 °C).
Uses
Propane is used in homes, business, industrial and agricultural, primarily for space heating, water heating and cooking. Propane gas has become a popular choice for barbecues and portable stoves because its low boiling point makes it vaporize as soon as it is released from its pressurized container. It appliances include space heaters, furnaces, water heaters, cooktops, ovens, clothes dryers and pool heaters.
Propane powers buses, forklifts, taxis, outboard boat motors, and ice resurfacing machines and is used for heat and cooking in recreational vehicles and campers. It is also used as fuel for internal combustion engine applications.
Information Source: