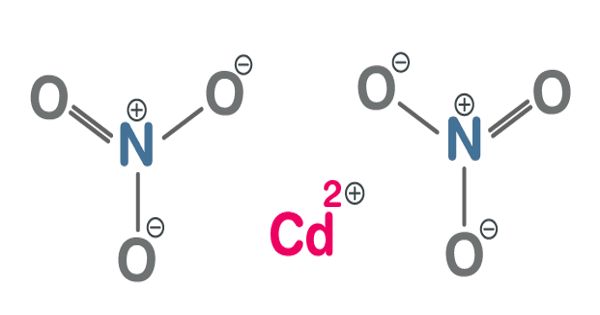
Cadmium Nitrate is a colorless, crystalline, inorganic compound that forms toxic fumes of cadmium oxides when heated. It describes any of the related members of a family of inorganic compounds with the general formula Cd(NO3)2.xH2O, the most commonly encountered form being the tetrahydrate. It is a cadmium salt and an inorganic nitrate salt. It is the source for Cadmium compatible with nitrates and lower pH value.
It is a white, hygroscopic crystal, soluble in water, alcohol, and liquid ammonia; used to give a reddish-yellow luster to glass and porcelain ware. The anhydrous form is volatile, but the others are colorless crystalline solids that are deliquescent, tending to absorb enough moisture from the air to form an aqueous solution. It has a role as a carcinogenic agent, a genotoxin and a hepatotoxic agent. Like other cadmium compounds, cadmium nitrate is known to be carcinogenic.
Properties
- Molecular Weight: 308.48
- Appearance: White Powder
- Melting Point: 59.5 °C
- Boiling Point: N/A
- Density: N/A
- Solubility in H2O: N/A
- Exact Mass: 309.921253
- Monoisotopic Mass: 309.921253

Preparation
Cadmium nitrate is a crystalline solid, soluble in water. It is prepared by dissolving cadmium metal or its oxide, hydroxide, or carbonate, in nitric acid followed by crystallization:
CdO + 2HNO3 → Cd(NO3)2 + H2O
CdCO + 2 HNO3 → Cd(NO3)2 + CO2 + H2O
Cd + 4 HNO3 → 2 NO2 + 2 H2O + Cd(NO3)2
Reactions
Thermal dissociation at elevated temperatures produces cadmium oxide and oxides of nitrogen. It has a role as a carcinogenic agent, a hepatotoxic agent and a genotoxin. When hydrogen sulfide is passed through an acidified solution of cadmium nitrate, yellow cadmium sulfide is formed. A red modification of the sulfide is formed under boiling conditions.
When with caustic soda solution, cadmium oxide forms precipitate of cadmium hydroxide. Many insoluble cadmium salts are obtained by such precipitation reactions.
Uses
Cadmium nitrate is used for coloring glass and porcelain and as a flash powder in photography. It is used in the production of cadmium hydroxide for use in alkaline batteries, to color glass and porcelain, in photography and in nuclear reactors.
Precaution
Exposure to this substance irritates the eyes, skin and respiratory tract and causes damage to the lungs resulting in shortness of breath, chest pain and pulmonary edema, and can also damage the kidneys causing proteinuria and decreased renal function. Inhalation of fumes can produce coughing, chest constriction, headache, nausea, vomiting, pneumonitis.
Information Source: